Enantioselective iridium-catalyzed carbonyl allylation from the alcohol or aldehyde oxidation level via transfer hydrogenative coupling of allyl acetate: departure from chirally modified allyl metal reagents in carbonyl addition
- PMID: 18841896
- PMCID: PMC2890235
- DOI: 10.1021/ja805722e
Enantioselective iridium-catalyzed carbonyl allylation from the alcohol or aldehyde oxidation level via transfer hydrogenative coupling of allyl acetate: departure from chirally modified allyl metal reagents in carbonyl addition
Abstract
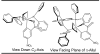
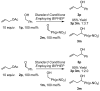
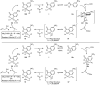
Under the conditions of transfer hydrogenation employing an iridium catalyst generated in situ from [Ir(cod)Cl]2, chiral phosphine ligand (R)-BINAP or (R)-Cl,MeO-BIPHEP, and m-nitrobenzoic acid, allyl acetate couples to allylic alcohols 1a-c, aliphatic alcohols 1d-l, and benzylic alcohols 1m-u to furnish products of carbonyl allylation 3a-u with exceptional levels of asymmetric induction. The very same set of optically enriched carbonyl allylation products 3a-u are accessible from enals 2a-c, aliphatic aldehydes 2d-l, and aryl aldehydes 2m-u, using iridium catalysts ligated by (-)-TMBTP or (R)-Cl,MeO-BIPHEP under identical conditions, but employing isopropanol as a hydrogen donor. A catalytically active cyclometallated complex V, which arises upon ortho-C-H insertion of iridium onto m-nitrobenzoic acid, was characterized by single-crystal X-ray diffraction. The results of isotopic labeling are consistent with intervention of symmetric iridium pi-allyl intermediates or rapid interconversion of sigma-allyl haptomers through the agency of a symmetric pi-allyl. Competition experiments demonstrate rapid and reversible hydrogenation-dehydrogenation of the carbonyl partner in advance of C-C coupling. However, the coupling products, which are homoallylic alcohols, experience very little erosion of optical purity by way of redox equilibration under the coupling conditions, although isopropanol, a secondary alcohol, may serve as terminal reductant. A plausible catalytic mechanism accounting for these observations is proposed, along with a stereochemical model that accounts for the observed sense of absolute stereoinduction. This protocol for asymmetric carbonyl allylation transcends the barriers imposed by oxidation level and the use of preformed allyl metal reagents.
Figures




References
-
-
For reviews on enantioselective carbonyl allylation, see: Yamamoto Y, Asao N. Chem. Rev. 1993;93:2207. Ramachandran PV. Aldrichim. Acta. 2002;35:23. Kennedy JWJ, Hall DG. Angew. Chem. Int. Ed. 2003;42:4732. Denmark SE, Fu J. Chem. Rev. 2003;103:2763. Yu C-M, Youn J, Jung H-K. Bull. Korean Chem. Soc. 2006;27:463. Marek I, Sklute G. Chem. Commun. 2007:1683. Hall DG. Synlett. 2007:1644.
-
-
- Mikhailov BM, Bubnov YN. Izv. Akad. Nauk SSSR, Ser. Khim. 1964:1874.
- Hosomi A, Sakurai H. Tetrahedron Lett. 1976;17:1295.
- Sakurai H. Pure Appl. Chem. 1982;54:1.
-
-
Selected examples of chirally modified allyl-metal reagents: Herold T, Hoffmann RW. Angew. Chem. Int. Ed. Engl. 1978;17:768. Hoffmann RW, Herold T. Chem. Ber. 1981;114:375. Hayashi T, Konishi M, Kumada M. J. Am. Chem. Soc. 1982;104:4963. Brown HC, Jadhav PK. J. Am. Chem. Soc. 1983;105:2092. Roush WR, Walts AE, Hoong LK. J. Am. Chem. Soc. 1985;107:8186. Reetz M. Pure Appl. Chem. 1988;60:1607. Short RP, Masamune S. J. Am. Chem. Soc. 1989;111:1892. Corey EJ, Yu C-M, Kim SS. J. Am. Chem. Soc. 1989:111–5495. Seebach D, Beck AK, Imwinkelzied R, Roggo S, Wonnacott A. Helv. Chim. Acta. 1987;70:954. Riediker M, Duthaler RO. Angew. Chem., Int. Ed. Engl. 1989;28:494. Panek JS, Yang M. J. Am. Chem. Soc. 1991;113:6594. Kinnaird JWA, Ng PY, Kubota K, Wang X, Leighton JL. J. Am. Chem. Soc. 2002;124:7920. Hackman BM, Lombardi PJ, Leighton JL. Org. Lett. 2004;6:4375. Burgos CH, Canales E, Matos K, Soderquist JA. J. Am. Chem. Soc. 2005;127:8044.
-
-
-
In Brown’s allylation protocol (reference 3d), the stoichiometric generation of isopinocampheol frequently complicates isolation of the allylation product: Ireland RE, Armstrong JD, III, Lebreton J, Meissner RS, Rizzacasa MA. J. Am. Chem. Soc. 1993;115:7152. Burova SA, McDonald FE. J. Am. Chem. Soc. 2004;126:2495. Ramachandran PV, Prabhudas B, Chandra JS, Reddy MVR. J. Org. Chem. 2004;69:6294. White JD, Hansen JD. J. Org. Chem. 2005;70:1963. Gao D, O’Doherty GA. Org. Lett. 2005;7:1069. Gao D, O’Doherty GA. J. Org. Chem. 2005;70:9932. Liu D, Xue J, Xie Z, Wei L, Zhang X, Li Y. Synlett. 2008:1526.
-
-
-
A notable exception involves the chirally modified allyl silanes developed by Leighton (reference 3l), for which highly efficient recovery of the chiral auxiliary is possible.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

