Hydrogen atom transfer reactions of a ruthenium imidazole complex: hydrogen tunneling and the applicability of the Marcus cross relation
- PMID: 18841973
- PMCID: PMC2633126
- DOI: 10.1021/ja805067h
Hydrogen atom transfer reactions of a ruthenium imidazole complex: hydrogen tunneling and the applicability of the Marcus cross relation
Abstract
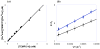
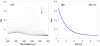
The reaction of Ru(II)(acac)2(py-imH) (Ru(II)imH) with TEMPO(*) (2,2,6,6-tetramethylpiperidine-1-oxyl radical) in MeCN quantitatively gives Ru(III)(acac)2(py-im) (Ru(III)im) and the hydroxylamine TEMPO-H by transfer of H(*) (H(+) + e(-)) (acac = 2,4-pentanedionato, py-imH = 2-(2'-pyridyl)imidazole). Kinetic measurements of this reaction by UV-vis stopped-flow techniques indicate a bimolecular rate constant k(3H) = 1400 +/- 100 M(-1) s(-1) at 298 K. The reaction proceeds via a concerted hydrogen atom transfer (HAT) mechanism, as shown by ruling out the stepwise pathways of initial proton or electron transfer due to their very unfavorable thermochemistry (Delta G(o)). Deuterium transfer from Ru(II)(acac)2(py-imD) (Ru(II)imD) to TEMPO(*) is surprisingly much slower at k(3D) = 60 +/- 7 M(-1) s(-1), with k(3H)/k(3D) = 23 +/- 3 at 298 K. Temperature-dependent measurements of this deuterium kinetic isotope effect (KIE) show a large difference between the apparent activation energies, E(a3D) - E(a3H) = 1.9 +/- 0.8 kcal mol(-1). The large k(3H)/k(3D) and DeltaE(a) values appear to be greater than the semiclassical limits and thus suggest a tunneling mechanism. The self-exchange HAT reaction between Ru(II)imH and Ru(III)im, measured by (1)H NMR line broadening, occurs with k(4H) = (3.2 +/- 0.3) x 10(5) M(-1) s(-1) at 298 K and k(4H)/k(4D) = 1.5 +/- 0.2. Despite the small KIE, tunneling is suggested by the ratio of Arrhenius pre-exponential factors, log(A(4H)/A(4D)) = -0.5 +/- 0.3. These data provide a test of the applicability of the Marcus cross relation for H and D transfers, over a range of temperatures, for a reaction that involves substantial tunneling. The cross relation calculates rate constants for Ru(II)imH(D) + TEMPO(*) that are greater than those observed: k(3H,calc)/k(3H) = 31 +/- 4 and k(3D,calc)/k(3D) = 140 +/- 20 at 298 K. In these rate constants and in the activation parameters, there is a better agreement with the Marcus cross relation for H than for D transfer, despite the greater prevalence of tunneling for H. The cross relation does not explicitly include tunneling, so close agreement should not be expected. In light of these results, the strengths and weaknesses of applying the cross relation to HAT reactions are discussed.
Figures




Similar articles
-
Nitroxyl radical plus hydroxylamine pseudo self-exchange reactions: tunneling in hydrogen atom transfer.J Am Chem Soc. 2009 Aug 26;131(33):11985-97. doi: 10.1021/ja904400d. J Am Chem Soc. 2009. PMID: 19618933 Free PMC article.
-
Trends in ground-state entropies for transition metal based hydrogen atom transfer reactions.J Am Chem Soc. 2009 Apr 1;131(12):4335-45. doi: 10.1021/ja8081846. J Am Chem Soc. 2009. PMID: 19275235 Free PMC article.
-
Synthesis and characterization of ruthenium bis(beta-diketonato) pyridine-imidazole complexes for hydrogen atom transfer.Inorg Chem. 2007 Dec 24;46(26):11190-201. doi: 10.1021/ic7015726. Epub 2007 Dec 1. Inorg Chem. 2007. PMID: 18052056 Free PMC article.
-
Proton-coupled electron transfer: a reaction chemist's view.Annu Rev Phys Chem. 2004;55:363-90. doi: 10.1146/annurev.physchem.55.091602.094446. Annu Rev Phys Chem. 2004. PMID: 15117257 Review.
-
Understanding Biological Hydrogen Transfer Through the Lens of Temperature Dependent Kinetic Isotope Effects.Acc Chem Res. 2018 Sep 18;51(9):1966-1974. doi: 10.1021/acs.accounts.8b00226. Epub 2018 Aug 28. Acc Chem Res. 2018. PMID: 30152685 Free PMC article. Review.
Cited by
-
Nitroxyl radical plus hydroxylamine pseudo self-exchange reactions: tunneling in hydrogen atom transfer.J Am Chem Soc. 2009 Aug 26;131(33):11985-97. doi: 10.1021/ja904400d. J Am Chem Soc. 2009. PMID: 19618933 Free PMC article.
-
Trends in ground-state entropies for transition metal based hydrogen atom transfer reactions.J Am Chem Soc. 2009 Apr 1;131(12):4335-45. doi: 10.1021/ja8081846. J Am Chem Soc. 2009. PMID: 19275235 Free PMC article.
-
Proton-Coupled Electron Transfer in Organic Synthesis: Fundamentals, Applications, and Opportunities.Top Curr Chem (Cham). 2016 Jun;374(3):30. doi: 10.1007/s41061-016-0030-6. Epub 2016 May 9. Top Curr Chem (Cham). 2016. PMID: 27573270 Free PMC article. Review.
-
Biochemistry and theory of proton-coupled electron transfer.Chem Rev. 2014 Apr 9;114(7):3381-465. doi: 10.1021/cr4006654. Epub 2014 Apr 1. Chem Rev. 2014. PMID: 24684625 Free PMC article. Review. No abstract available.
-
Diazaphosphinanes as hydride, hydrogen atom, proton or electron donors under transition-metal-free conditions: thermodynamics, kinetics, and synthetic applications.Chem Sci. 2020 Mar 5;11(14):3672-3679. doi: 10.1039/c9sc05883d. Chem Sci. 2020. PMID: 34094055 Free PMC article.
References
-
- Hynes JT, Klinman JP, Limbach H-H, Schowen RL, editors. Hydrogen-Transfer Reactions. Wiley-VCH; Weinheim: 2007.
-
- Olah GA, Molnár Á. Hydrocarbon Chemistry. 2. Wiley; Hoboken, NJ: 2003.
- Sheldon RA, Kochi JK. Metal-Catalyzed Oxidations of Organic Compounds. Academic Press; New York: 1981.
- Kochi JK. Free Radicals. Wiley; New York: 1973.
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford University Press; Oxford: 1999.
- Fossey J, Lefort D, Sorba J. Free Radicals in Organic Chemistry. Wiley; New York: 1995.
- Lazár M, Rychlý J, Klimo V, Pelikán P, Valko L. Free Radicals in Chemistry and Biology. CRC Press; Boca Raton, Fl: 1989.
-
-
For recent references, see Gansäuer A, Fan CA, Piestert F. J Am Chem Soc. 2008;130:6916.Nieto I, Ding F, Bontchev RP, Wang H, Smith JM. J Am Chem Soc. 2008;130:2716.Maiti D, Lee D-H, Gaoutchenova K, Würtele C, Holthausen MC, Narducci Sarjeant AA, Sundermeyer J, Schindler S, Karlin KD. Angew Chem Int Ed. 2008;47:82.Lam WWY, Man WL, Leung CF, Wong CY, Lau TC. J Am Chem Soc. 2007;129:13646.Zdilla MJ, Dexheimer JL, Abu-Omar MM. J Am Chem Soc. 2007;129:11505.Choi J, Tang L, Norton JR. J Am Chem Soc. 2007;129:234.Vasbinder MJ, Bakac A. Inorg Chem. 2007;46:2921.Zhang J, Grills DC, Huang KW, Fujita E, Bullock RM. J Am Chem Soc. 2005;127:15684.
-
-
- Mayer JM. Annu Rev Phys Chem. 2004;55:363. - PubMed
- Mayer JM, Rhile IJ. Biochim Biophys Acta. 2004;1655:51. - PubMed
- Mayer JM, Rhile IJ, Larsen FB, Mader EA, Markle TF, DiPasquale AG. Photosynth Res. 2006;87:3. - PubMed
- Mayer JM, Mader EA, Roth JP, Bryant JR, Matsuo T, Dehestani A, Bales BC, Watson EJ, Osako T, Valliant-Saunders K, Lam W-H, Hrovat DA, Borden WT, Davidson ER. J Mol Catal A: Chem. 2006;251:24.
- Isborn C, Hrovat DA, Borden WT, Mayer JM, Carpenter BK. J Am Chem Soc. 2005;127:5794. - PubMed
-
- Warren JJ, Mayer JM. J Am Chem Soc. 2008;130:2774–2776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

