O-phospho-L-serine and the thiocarboxylated sulfur carrier protein CysO-COSH are substrates for CysM, a cysteine synthase from Mycobacterium tuberculosis
- PMID: 18842002
- PMCID: PMC2647513
- DOI: 10.1021/bi8013664
O-phospho-L-serine and the thiocarboxylated sulfur carrier protein CysO-COSH are substrates for CysM, a cysteine synthase from Mycobacterium tuberculosis
Abstract
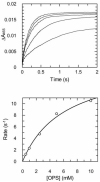
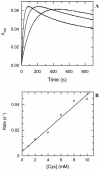
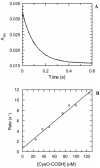
The kinetic pathway of CysM, a cysteine synthase from Mycobacterium tuberculosis, was studied by transient-state kinetic techniques. The expression of which is upregulated under conditions of oxidative stress. This enzyme exhibits extensive homology with the B-isozymes of the well-studied O-acetylserine sulfhydrylase family and employs a similar chemical mechanism involving a stable alpha-aminoacrylate intermediate. However, we show that specificity of CysM for its amino acid substrate is more than 500-fold greater for O-phospho-L-serine than for O-acetyl-L-serine, suggesting that O-phospho-L-serine is the likely substrate in vivo. We also investigated the kinetics of the carbon-sulfur bond-forming reaction between the CysM-bound alpha-aminoacrylate intermediate and the thiocarboxylated sulfur carrier protein, CysO-COSH. The specificity of CysM for this physiological sulfide equivalent is more than 3 orders of magnitude greater than that for bisulfide. Moreover, the kinetics of this latter reaction are limited by association of the proteins, while the reaction with bisulfide is consistent with a rapid equilibrium binding model. We interpret this finding to suggest that the CysM active site with the bound aminoacrylate intermediate is protected from solvent and that binding of CysO-COSH produces a conformational change allowing rapid sulfur transfer. This study represents the first detailed kinetic characterization of sulfide transfer from a sulfide carrier protein.
Figures





Similar articles
-
Cysteine synthase (CysM) of Mycobacterium tuberculosis is an O-phosphoserine sulfhydrylase: evidence for an alternative cysteine biosynthesis pathway in mycobacteria.J Biol Chem. 2008 Nov 14;283(46):31567-74. doi: 10.1074/jbc.M804877200. Epub 2008 Sep 16. J Biol Chem. 2008. PMID: 18799456
-
Crystal structure of a sulfur carrier protein complex found in the cysteine biosynthetic pathway of Mycobacterium tuberculosis.Biochemistry. 2008 Sep 30;47(39):10354-64. doi: 10.1021/bi800915j. Epub 2008 Sep 5. Biochemistry. 2008. PMID: 18771296 Free PMC article.
-
The C-terminal of CysM from Mycobacterium tuberculosis protects the aminoacrylate intermediate and is involved in sulfur donor selectivity.FEBS Lett. 2009 Jan 22;583(2):330-6. doi: 10.1016/j.febslet.2008.12.019. Epub 2008 Dec 26. FEBS Lett. 2009. PMID: 19101553
-
Structure and mechanism of O-acetylserine sulfhydrylase.J Biol Chem. 2004 Jun 25;279(26):26803-6. doi: 10.1074/jbc.R400001200. Epub 2004 Apr 8. J Biol Chem. 2004. PMID: 15073190 Review.
-
Synthesis of L-cysteine in Salmonella typhimurium.Ciba Found Symp. 1979;(72):87-99. doi: 10.1002/9780470720554.ch6. Ciba Found Symp. 1979. PMID: 398768 Review.
Cited by
-
The regulation of sulfur metabolism in Mycobacterium tuberculosis.PLoS Pathog. 2011 Jul;7(7):e1002036. doi: 10.1371/journal.ppat.1002036. Epub 2011 Jul 21. PLoS Pathog. 2011. PMID: 21811406 Free PMC article. Review.
-
Structural insight into amino group-carrier protein-mediated lysine biosynthesis: crystal structure of the LysZ·LysW complex from Thermus thermophilus.J Biol Chem. 2015 Jan 2;290(1):435-47. doi: 10.1074/jbc.M114.595983. Epub 2014 Nov 12. J Biol Chem. 2015. PMID: 25392000 Free PMC article.
-
Ubiquitin-like proteins and their roles in archaea.Trends Microbiol. 2013 Jan;21(1):31-8. doi: 10.1016/j.tim.2012.09.006. Epub 2012 Nov 8. Trends Microbiol. 2013. PMID: 23140889 Free PMC article. Review.
-
Archaeal proteasomes and sampylation.Subcell Biochem. 2013;66:297-327. doi: 10.1007/978-94-007-5940-4_11. Subcell Biochem. 2013. PMID: 23479445 Free PMC article. Review.
-
Edwardsiella tarda Hfq: impact on host infection and global protein expression.Vet Res. 2014 Feb 25;45(1):23. doi: 10.1186/1297-9716-45-23. Vet Res. 2014. PMID: 24568370 Free PMC article.
References
-
- Scientific Working Group Report on Tuberculosis. World Health Organization; Geneva: 2006.
-
- Kredich NM, Tomkins GM. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J. Biol. Chem. 1966;241:4955–4965. - PubMed
-
- Johnson CM, Roderick SL, Cook PF. The serine acetyltransferase reaction: acetyl transfer from an acylpantothenyl donor to an alcohol. Arch. Biochem. Biophys. 2007;433:85–95. - PubMed
-
- Tai C-H, Cook PF. Pyridoxal-5′-phosphate-dependent α,β-elimination reactions: Mechanism of O-acetylserine sulfhydrylase. Acc. Chem. Res. 2001;34:49–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

