Substituent effects on the rearrangements of cyclohexyl to cyclopentyl radicals involving avermectin-related radicals
- PMID: 18842059
- PMCID: PMC2652414
- DOI: 10.1021/jo800923a
Substituent effects on the rearrangements of cyclohexyl to cyclopentyl radicals involving avermectin-related radicals
Abstract
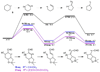
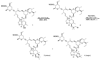
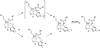
The rearrangement of a substituted cyclohexyl radical to a cyclopentylmethyl radical on the skeleton of avermectin B1 has been investigated using density functional (UB3LYP/6-31G(d)) and G3MP2B3 computational methods. The rearrangement is preferred when highly radical stabilizing groups are present at the 2- and 3-positions of the cyclohexyl radical. A substituent on the 3-position of the cyclohexyl radical enables ring-cleavage of the cyclohexyl radical, while a radical stabilizing substituent on the 2-position of the cyclohexyl radical stabilizes the final cyclopentylmethyl radical, enabling the overall rearrangement and reversing the normal thermodynamic preference for the hexenyl radical ring closure.
Figures



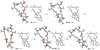


Similar articles
-
Rearrangement of a cyclohexyl radical to a cyclopentylmethyl radical on the avermectin skeleton.Org Lett. 2008 Jun 5;10(11):2255-8. doi: 10.1021/ol8001283. Epub 2008 May 8. Org Lett. 2008. PMID: 18461948
-
Theoretical elucidation of kinetic and thermodynamic control of radical addition regioselectivity.J Am Chem Soc. 2003 Apr 9;125(14):4271-8. doi: 10.1021/ja029342q. J Am Chem Soc. 2003. PMID: 12670249
-
Synthesis and anticancer evaluation of naphthoquinone esters with 2'-cyclopentyl and 2'-cyclohexyl substituents.Biosci Biotechnol Biochem. 2010;74(6):1205-14. doi: 10.1271/bbb.90946. Epub 2010 Jun 7. Biosci Biotechnol Biochem. 2010. PMID: 20530913
-
Fragrance material review on cyclohexyl cyclopent-2-ene-1-acetate.Food Chem Toxicol. 2008 Dec;46 Suppl 12:S56-7. doi: 10.1016/j.fct.2008.09.034. Epub 2008 Sep 19. Food Chem Toxicol. 2008. PMID: 18845209 Review.
-
New ventures in the chemistry of avermectins.Bioorg Med Chem. 2009 Jun 15;17(12):4085-95. doi: 10.1016/j.bmc.2008.12.069. Epub 2009 Jan 6. Bioorg Med Chem. 2009. PMID: 19168364 Review.
Cited by
-
Origins of stereoselectivity in the trans Diels-Alder paradigm.J Am Chem Soc. 2010 Jul 14;132(27):9335-40. doi: 10.1021/ja1009162. J Am Chem Soc. 2010. PMID: 20557046 Free PMC article.
-
Factors Controlling Diastereoselectivity and Reactivity in the Catalytic Aerobic Carbooxygenation of (E)-2-Fluoro-3-aryl-allyl Nitroacetates.Chem Asian J. 2025 Jul;20(13):e202500336. doi: 10.1002/asia.202500336. Epub 2025 May 2. Chem Asian J. 2025. PMID: 40318140 Free PMC article.
References
-
- Inoue M, Miyazaki K, Ishihara Y, Tatami A, Ohnuma Y, Kawada Y, Komano K, Yamashita S, Lee N, Hirama M. J. Am. Chem. Soc. 2006;128:9352–9354. - PubMed
- Fishlock D, Williams RM. Org. Lett. 2006;8:3299–3301. - PubMed
- Tangirala R, Antony S, Agama K, Pommier Y, Curran DP. Synlett. 2005;18:2843–2846.
- Trost BM, Shen HC, Surivet J-P. J. Am. Chem. Soc. 2004;126:12565–12579. - PubMed
- Ng D, Yang Z, Garcia-Garibay MA. Org. Lett. 2004;6:645–647. - PubMed
- Takeda Y, Nakabayashi T, Shirai A, Fukumoto D, Kiguchi T, Naito T. Tetrahedron Lett. 2004;45:3481–3484.
- Trost BM, Shen HC, Surivet J-P. Angew. Chem. Int. Ed. 2003;42:3943–3947. - PubMed
- Inoue M, Sato T, Hirama M. J. Am. Chem. Soc. 2003;125:10772–10773. - PubMed
- White JD, Blakemore PR, Green NJ, Hauser EB, Holoboski MA, Keown LE, Kolz CSN, Phillips BW. J. Org. Chem. 2002;67:7750–7760. - PubMed
- Gilbert BC, Parsons AF. J. Chem Soc., Perkin Trans. 2. 2002;3:367–387.
- Fischer H. Chem. Rev. 2001;101:3581–3610. - PubMed
- Takeda Y, Nagano H, Matsuda M, Yajima T. J. Chem Soc., Perkin Trans. 1. 2001;2:174–182.
- Murphy JA. Pure Appl. Chem. 2000;72:1327–1334.
- Belval F, Fruchier A, Chavis C, Montero J-L, Lucas M. J. Chem. Soc., Perkin Trans. 1. 1999:697–703.
- Ohtsuka M, Takekawa Y, Shishido K. Tetrahedron Lett. 1998;39:5803–5806.
- Robertson J, Burrows JN, Stupple PA. Tetrahedron. 1997;53:14807–14820.
- Bogen S, Malacria M. J. Am. Chem. Soc. 1996;118:3992–3993.
- Tamao K, Nagata K, Ito Y, Maeda K, Shiro M. Synlett. 1994:257–259.
- Journet M, Malacria M. J. Org. Chem. 1992;57:3085–3093.
- Baldwin JE, Adlington RM, Robertson J. Tetrahedron. 1991;47:6795–6812.
- Journet M, Smadja W, Malacria M. Synlett. 1990;6:320–322.
- Journet M, Magnol E, Angel G, Malacria M. Tetrahedron Lett. 1990;31:4445–4448.
- Baldwin JE, Adlington RM, Robertson J. Tetrahedron. 1989;45:909–922.
- Baldwin JE, Adlington RM, Robertson J. J. Chem. Soc., Chem. Commun. 1988;21:1404–1406.
-
- Pedersen JM, Bowman WR, Elsegood MR, Fletcher AJ, Lovell PJ. J. Org. Chem. 2005;70:10615–10618. - PubMed
- Justicia J, Oller-Lopez JL, Campana AG, Oltra JE, Cuerva JM, Bunuel E, Cardenas DJ. J. Am. Chem. Soc. 2005;127:14911–14921. - PubMed
- Nicolaou KC, Sasmal PK, Koftis TV, Converso A, Loizidou E, Kaiser F, Roecker AJ, Dellios CC, Sun X-W, Petrovic G. Angew. Chem. Int. Ed. 2005;44:3447–3452. - PubMed
- Kovalenko SV, Peabody S, Manoharan M, Clark RJ, Alabugin IV. Org. Lett. 2004;6:2457–2460. - PubMed
- Miyabe H, Ueda M, Fujii, Kayoko N, Azusa, Naito T. J. Org. Chem. 2003;68:5618–5626. - PubMed
- Blaszykowski C, Dhimare A-L, Fenterbank L, Malacria M. Org. Lett. 2003;5:1341–1344. - PubMed
- Miyabe H, Ueda M, Nishimura A, Naito T. Org. Lett. 2002;4:131–134. - PubMed
- Gansauer A, Pierobon M, Bluhm H. Angew. Chem. Int. Ed. 2002;41:3206–3208. - PubMed
- Cointeaux L, Berrien J-F, Mayrargue J. Tetrahedron Lett. 2002;43:6275–6277.
- Friedstad GK, Massari SE. Org. Lett. 2000;2:4237–4240. - PubMed
- Friedstad GK. Org. Lett. 1999;1:1499–1501.
- Stork G, Mook R., Jr J. Am. Chem. Soc. 1983;105:3720–3722.
-
- Bennasar M-L, Roca T, Ferrando F. J. Org. Chem. 2006;71:1746–1749. - PubMed
- Majumdar KC, Muhuri S. Synthesis. 2006;16:2725–2730.
- Bermejo FA, Mateos AF, Escribano AM, Lago RM, Burón LM, López MR, González RR. Tetrahedron. 2006;62:8933–8942.
- Pianowski Z, Rupnicki L, Cmoch P, Staliński K. Synlett. 2005;6:900–904.
- Szpilman AM, Korshin EE, Rozenberg H, Bachi MD. J. Org. Chem. 2005;70:3618–3632. - PubMed
- Lee H-Y, Moon DK, Bahn JS. Tetrahedron Lett. 2005;46:1455–1458.
- Rashatasakhon P, Ozdemir AD, Willis J, Padwa A. Org. Lett. 2004;6:917–920. - PubMed
- Pave G, Usse-Versluys S, Viaud-Massuard M-C, Guillaumet G. Org. Let. 2003;5:4253–4256. - PubMed
- Vargas AC, Quiclet-Sire B, Zard SZ. Org. Lett. 2003;5:3717–3719. - PubMed
- Sumi S, Matsumoto K, Tokuyama H, Fukuyama T. Org. Lett. 2003;5:1891–1893. - PubMed
- Enholm EJ, Cottone JS, Allais F. Org. Lett. 2001;3:145–147. - PubMed
- Gauthier DR, Zandi KS, Shea KJ. Tetrahedron. 1998;54:2289–2338.
-
- Springer JP, Arison BH, Hirshfield JM, Hoogsteen K. J. Am. Chem. Soc. 1981;103:4221.
- Fischer MH, Mrozik H. Macrolide Antibiotics. Academic Press; 1985. p. 553.
- Davies HG, Green RH. Nat. Prod Rep. 1986;3:87. - PubMed
-
- Kessabi FM, Winkler T, Luft JAR, Houk KN. Org. Lett. 2008 ASAP. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

