SLC2A9 is a high-capacity urate transporter in humans
- PMID: 18842065
- PMCID: PMC2561076
- DOI: 10.1371/journal.pmed.0050197
SLC2A9 is a high-capacity urate transporter in humans
Abstract
Background: Serum uric acid levels in humans are influenced by diet, cellular breakdown, and renal elimination, and correlate with blood pressure, metabolic syndrome, diabetes, gout, and cardiovascular disease. Recent genome-wide association scans have found common genetic variants of SLC2A9 to be associated with increased serum urate level and gout. The SLC2A9 gene encodes a facilitative glucose transporter, and it has two splice variants that are highly expressed in the proximal nephron, a key site for urate handling in the kidney. We investigated whether SLC2A9 is a functional urate transporter that contributes to the longstanding association between urate and blood pressure in man.
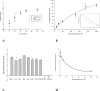
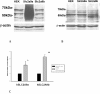
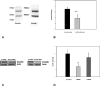
Methods and findings: We expressed both SLC2A9 splice variants in Xenopus laevis oocytes and found both isoforms mediate rapid urate fluxes at concentration ranges similar to physiological serum levels (200-500 microM). Because SLC2A9 is a known facilitative glucose transporter, we also tested whether glucose or fructose influenced urate transport. We found that urate is transported by SLC2A9 at rates 45- to 60-fold faster than glucose, and demonstrated that SLC2A9-mediated urate transport is facilitated by glucose and, to a lesser extent, fructose. In addition, transport is inhibited by the uricosuric benzbromarone in a dose-dependent manner (Ki = 27 microM). Furthermore, we found urate uptake was at least 2-fold greater in human embryonic kidney (HEK) cells overexpressing SLC2A9 splice variants than nontransfected kidney cells. To confirm that our findings were due to SLC2A9, and not another urate transporter, we showed that urate transport was diminished by SLC2A9-targeted siRNA in a second mammalian cell line. In a cohort of men we showed that genetic variants of SLC2A9 are associated with reduced urinary urate clearance, which fits with common variation at SLC2A9 leading to increased serum urate. We found no evidence of association with hypertension (odds ratio 0.98, 95% confidence interval [CI] 0.9 to 1.05, p > 0.33) by meta-analysis of an SLC2A9 variant in six case-control studies including 11,897 participants. In a separate meta-analysis of four population studies including 11,629 participants we found no association of SLC2A9 with systolic (effect size -0.12 mm Hg, 95% CI -0.68 to 0.43, p = 0.664) or diastolic blood pressure (effect size -0.03 mm Hg, 95% CI -0.39 to 0.31, p = 0.82).
Conclusions: This study provides evidence that SLC2A9 splice variants act as high-capacity urate transporters and is one of the first functional characterisations of findings from genome-wide association scans. We did not find an association of the SLC2A9 gene with blood pressure in this study. Our findings suggest potential pathogenic mechanisms that could offer a new drug target for gout.
Conflict of interest statement
Figures


Similar articles
-
Mouse GLUT9: evidences for a urate uniporter.Am J Physiol Renal Physiol. 2009 Sep;297(3):F612-9. doi: 10.1152/ajprenal.00139.2009. Epub 2009 Jul 8. Am J Physiol Renal Physiol. 2009. PMID: 19587147
-
Human SLC2A9a and SLC2A9b isoforms mediate electrogenic transport of urate with different characteristics in the presence of hexoses.Am J Physiol Renal Physiol. 2012 Aug 15;303(4):F527-39. doi: 10.1152/ajprenal.00134.2012. Epub 2012 May 30. Am J Physiol Renal Physiol. 2012. PMID: 22647630 Free PMC article.
-
SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout.Nat Genet. 2008 Apr;40(4):437-42. doi: 10.1038/ng.106. Epub 2008 Mar 9. Nat Genet. 2008. PMID: 18327257
-
Solute carrier family 2, member 9 and uric acid homeostasis.Curr Opin Nephrol Hypertens. 2009 Sep;18(5):428-32. doi: 10.1097/MNH.0b013e32832ee3de. Curr Opin Nephrol Hypertens. 2009. PMID: 19593129 Review.
-
A 'complexity' of urate transporters.Kidney Int. 2010 Sep;78(5):446-52. doi: 10.1038/ki.2010.206. Epub 2010 Jul 7. Kidney Int. 2010. PMID: 20613716 Review.
Cited by
-
Research progress of risk factors and early diagnostic biomarkers of gout-induced renal injury.Front Immunol. 2022 Sep 20;13:908517. doi: 10.3389/fimmu.2022.908517. eCollection 2022. Front Immunol. 2022. PMID: 36203589 Free PMC article. Review.
-
Acute kidney injury in two children caused by renal hypouricaemia type 2.Pediatr Nephrol. 2012 Aug;27(8):1411-5. doi: 10.1007/s00467-012-2174-0. Epub 2012 Apr 21. Pediatr Nephrol. 2012. PMID: 22527535
-
Contribution of uric acid to cancer risk, recurrence, and mortality.Clin Transl Med. 2012 Aug 15;1(1):16. doi: 10.1186/2001-1326-1-16. Clin Transl Med. 2012. PMID: 23369448 Free PMC article.
-
Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy.Proc Natl Acad Sci U S A. 2009 Sep 8;106(36):15501-6. doi: 10.1073/pnas.0904411106. Epub 2009 Aug 21. Proc Natl Acad Sci U S A. 2009. PMID: 19706426 Free PMC article.
-
Urate transport in health and disease.Best Pract Res Clin Rheumatol. 2021 Dec;35(4):101717. doi: 10.1016/j.berh.2021.101717. Epub 2021 Oct 21. Best Pract Res Clin Rheumatol. 2021. PMID: 34690083 Free PMC article. Review.
References
-
- Cannon PJ, Stason WB, Demartini FE, Sommers SC, Laragh JH. Hyperuricemia in primary and renal hypertension. N Engl J Med. 1966;275:457–464. - PubMed
-
- Sundstrom J, Sullivan L, D'Agostino RB, Levy D, Kannel WB, et al. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45:28–33. - PubMed
-
- Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, et al. Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003;18:523–530. - PubMed
-
- Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–2410. - PubMed
-
- Anzai N, Kanai Y, Endou H. New insights into renal transport of urate. Curr Opin Rheumatol. 2007;19:151–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G9521010/MRC_/Medical Research Council/United Kingdom
- G0400874/MRC_/Medical Research Council/United Kingdom
- R01 HL036310/HL/NHLBI NIH HHS/United States
- G19/35/MRC_/Medical Research Council/United Kingdom
- G0100222/MRC_/Medical Research Council/United Kingdom
- R37 AG013196/AG/NIA NIH HHS/United States
- G9521010D/MRC_/Medical Research Council/United Kingdom
- T32 DK007120/DK/NIDDK NIH HHS/United States
- R01 AG013196/AG/NIA NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- 076113/B/04/Z/WT_/Wellcome Trust/United Kingdom
- RG/07/008/23674/BHF_/British Heart Foundation/United Kingdom
- G8802774/MRC_/Medical Research Council/United Kingdom
- T32-DK0712/DK/NIDDK NIH HHS/United States
- PG02/128/BHF_/British Heart Foundation/United Kingdom
- R01 HD040390/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

