Global transcriptional response to mammalian temperature provides new insight into Francisella tularensis pathogenesis
- PMID: 18842136
- PMCID: PMC2576331
- DOI: 10.1186/1471-2180-8-172
Global transcriptional response to mammalian temperature provides new insight into Francisella tularensis pathogenesis
Abstract
Background: After infecting a mammalian host, the facultative intracellular bacterium, Francisella tularensis, encounters an elevated environmental temperature. We hypothesized that this temperature change may regulate genes essential for infection.
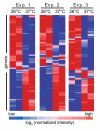
Results: Microarray analysis of F. tularensis LVS shifted from 26 degrees C (environmental) to 37 degrees C (mammalian) showed approximately 11% of this bacterium's genes were differentially-regulated. Importantly, 40% of the protein-coding genes that were induced at 37 degrees C have been previously implicated in virulence or intracellular growth of Francisella in other studies, associating the bacterial response to this temperature shift with pathogenesis. Forty-four percent of the genes induced at 37 degrees C encode proteins of unknown function, suggesting novel Francisella virulence traits are regulated by mammalian temperature. To explore this possibility, we generated two mutants of loci induced at 37 degrees C [FTL_1581 and FTL_1664 (deoB)]. The FTL_1581 mutant was attenuated in a chicken embryo infection model, which was likely attributable to a defect in survival within macrophages. FTL_1581 encodes a novel hypothetical protein that we suggest naming temperature-induced, virulence-associated locus A, tivA. Interestingly, the deoB mutant showed diminished entry into mammalian cells compared to wild-type LVS, including primary human macrophages and dendritic cells, the macrophage-like RAW 264.7 line, and non-phagocytic HEK-293 cells. This is the first study identifying a Francisella gene that contributes to uptake into both phagocytic and non-phagocytic host cells.
Conclusion: Our results provide new insight into mechanisms of Francisella virulence regulation and pathogenesis. F. tularensis LVS undergoes considerable gene expression changes in response to mammalian body temperature. This temperature shift is important for the regulation of genes that are critical for the pathogenesis of Francisella. Importantly, the compilation of temperature-regulated genes also defines a rich collection of novel candidate virulence determinants, including tivA (FTL_1581). An analysis of tivA and deoB (FTL_1664) revealed that these genes contribute to intracellular survival and entry into mammalian cells, respectively.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

