G4 resolvase 1 binds both DNA and RNA tetramolecular quadruplex with high affinity and is the major source of tetramolecular quadruplex G4-DNA and G4-RNA resolving activity in HeLa cell lysates
- PMID: 18842585
- PMCID: PMC2596407
- DOI: 10.1074/jbc.M806277200
G4 resolvase 1 binds both DNA and RNA tetramolecular quadruplex with high affinity and is the major source of tetramolecular quadruplex G4-DNA and G4-RNA resolving activity in HeLa cell lysates
Abstract
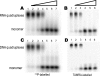
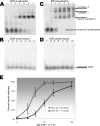
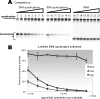
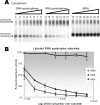
Quadruplex structures that result from stacking of guanine quartets in nucleic acids possess such thermodynamic stability that their resolution in vivo is likely to require specific recognition by specialized enzymes. We previously identified the major tetramolecular quadruplex DNA resolving activity in HeLa cell lysates as the gene product of DHX36 (Vaughn, J. P., Creacy, S. D., Routh, E. D., Joyner-Butt, C., Jenkins, G. S., Pauli, S., Nagamine, Y., and Akman, S. A. (2005) J. Biol Chem. 280, 38117-38120), naming the enzyme G4 Resolvase 1 (G4R1). G4R1 is also known as RHAU, an RNA helicase associated with the AU-rich sequence of mRNAs. We now show that G4R1/RHAU binds to and resolves tetramolecular RNA quadruplex as well as tetramolecular DNA quadruplex structures. The apparent K(d) values of G4R1/RHAU for tetramolecular RNA quadruplex and tetramolecular DNA quadruplex were exceptionally low: 39 +/- 6 and 77 +/- 6 Pm, respectively, as measured by gel mobility shift assay. In competition studies tetramolecular RNA quadruplex structures inhibited tetramolecular DNA quadruplex structure resolution by G4R1/RHAU more efficiently than tetramolecular DNA quadruplex structures inhibited tetramolecular RNA quadruplex structure resolution. Down-regulation of G4R1/RHAU in HeLa T-REx cells by doxycycline-inducible short hairpin RNA caused an 8-fold loss of RNA and DNA tetramolecular quadruplex resolution, consistent with G4R1/RHAU representing the major tetramolecular quadruplex helicase activity for both RNA and DNA structures in HeLa cells. This study demonstrates for the first time the RNA quadruplex resolving enzymatic activity associated with G4R1/RHAU and its exceptional binding affinity, suggesting a potential novel role for G4R1/RHAU in targeting in vivo RNA quadruplex structures.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

