Human immunodeficiency virus type 1 superinfection occurs despite relatively robust neutralizing antibody responses
- PMID: 18842728
- PMCID: PMC2593335
- DOI: 10.1128/JVI.01730-08
Human immunodeficiency virus type 1 superinfection occurs despite relatively robust neutralizing antibody responses
Abstract
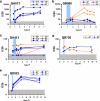
Superinfection by a second human immunodeficiency virus type 1 (HIV-1) strain indicates that gaps in protective immunity occur during natural infection. To define the role of HIV-1-specific neutralizing antibodies (NAbs) in this setting, we examined NAb responses in 6 women who became superinfected between approximately 1 to 5 years following initial infection compared to 18 women with similar risk factors who did not. Although superinfected individuals had less NAb breadth than matched controls at approximately 1 year postinfection, no significant differences in the breadth or potency of NAb responses were observed just prior to the second infection. In fact, four of the six subjects had relatively broad and potent NAb responses prior to infection by the second strain. To more specifically examine the specificity of the NAbs against the superinfecting virus, these variants were cloned from five of the six individuals. The superinfecting variants did not appear to be inherently neutralization resistant, as measured against a pool of plasma from unrelated HIV-infected individuals. Moreover, the superinfected individuals were able to mount autologous NAb responses to these variants following reinfection. In addition, most superinfected individuals had NAbs that could neutralize their second viral strains prior to their reinfection, suggesting that the level of NAbs elicited during natural infection was not sufficient to block infection. These data indicate that preventing infection by vaccination will likely require broader and more potent NAb responses than those found in HIV-1-infected individuals.
Figures




References
-
- Altfeld, M., T. M. Allen, X. G. Yu, M. N. Johnston, D. Agrawal, B. T. Korber, D. C. Montefiori, D. H. O'Connor, B. T. Davis, P. K. Lee, E. L. Maier, J. Harlow, P. J. Goulder, C. Brander, E. S. Rosenberg, and B. D. Walker. 2002. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature 420434-439. - PubMed
-
- Beirnaert, E., P. Nyambi, B. Willems, L. Heyndrickx, R. Colebunders, W. Janssens, and G. van der Groen. 2000. Identification and characterization of sera from HIV-infected individuals with broad cross-neutralizing activity against group M (Env clade A-H) and group O primary HIV-1 isolates. J. Med. Virol. 6214-24. - PubMed
-
- Blish, C., R. Nedellec, K. Mandaliya, D. Mosier, and J. Overbaugh. 2007. HIV-1 subtype A envelope variants from early in infection have variable sensitivity to neutralization and to inhibitors of viral entry. AIDS 6693-702. - PubMed
-
- Bocher, W. O., S. Herzog-Hauff, W. Herr, K. Heermann, G. Gerken, K. H. Meyer Zum Buschenfelde, and H. F. Lohr. 1996. Regulation of the neutralizing anti-hepatitis B surface (HBs) antibody response in vitro in HBs vaccine recipients and patients with acute or chronic hepatitis B virus (HBV) infection. Clin. Exp. Immunol. 10552-58. - PMC - PubMed
-
- Brown, S. A., J. L. Hurwitz, X. Zhan, P. C. Doherty, and K. S. Slobod. 2005. CD8+ T-cells: are they sufficient to prevent, contain or eradicate HIV-1 infection? Curr. Drug Targets Infect. Disord. 5113-119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

