Llama antibody fragments with cross-subtype human immunodeficiency virus type 1 (HIV-1)-neutralizing properties and high affinity for HIV-1 gp120
- PMID: 18842738
- PMCID: PMC2593325
- DOI: 10.1128/JVI.01379-08
Llama antibody fragments with cross-subtype human immunodeficiency virus type 1 (HIV-1)-neutralizing properties and high affinity for HIV-1 gp120
Abstract
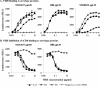
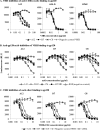
Members of the Camelidae family produce immunoglobulins devoid of light chains. We have characterized variable domains of these heavy chain antibodies, the VHH, from llamas immunized with human immunodeficiency virus type 1 (HIV-1) envelope protein gp120 in order to identify VHH that can inhibit HIV-1 infection. To increase the chances of isolating neutralizing VHH, we employed a functional selection approach, involving panning of phage libraries expressing the VHH repertoire on recombinant gp120, followed by a competitive elution with soluble CD4. By immunizing with gp120 derived from an HIV-1 subtype B'/C primary isolate, followed by panning on gp120 from HIV-1 isolates of subtypes A, B, and C, we could select for VHH with cross-subtype neutralizing activity. Three VHH able to neutralize HIV-1 primary isolates of subtypes B and C were characterized. These bound to recombinant gp120 with affinities close to the suggested affinity ceiling for in vivo-maturated antibodies and competed with soluble CD4 for this binding, indicating that their mechanism of neutralization involves interacting with the functional envelope spike prior to binding to CD4. The most potent VHH in terms of low 50% inhibitory concentration (IC(50)) and IC(90) values and cross-subtype reactivity was A12. These results indicate that camelid VHH can be potent HIV-1 entry inhibitors. Since VHH are stable and can be produced at a relatively low cost, they may be considered for applications such as HIV-1 microbicide development. Antienvelope VHH might also prove useful in defining neutralizing and nonneutralizing epitopes on HIV-1 envelope proteins, with implications for HIV-1 vaccine design.
Figures



References
-
- Aasa-Chapman, M. M., A. Hayman, P. Newton, D. Cornforth, I. Williams, P. Borrow, P. Balfe, and A. McKnight. 2004. Development of the antibody response in acute HIV-1 infection. AIDS 18371-381. - PubMed
-
- Baral, T. N., S. Magez, B. Stijlemans, K. Conrath, B. Vanhollebeke, E. Pays, S. Muyldermans, and P. De Baetselier. 2006. Experimental therapy of African trypanosomiasis with a nanobody-conjugated human trypanolytic factor. Nat. Med. 12580-584. - PubMed
-
- Barbas, C. F., III, D. Hu, N. Dunlop, L. Sawyer, D. Cababa, R. M. Hendry, P. L. Nara, and D. R. Burton. 1994. In vitro evolution of a neutralizing human antibody to human immunodeficiency virus type 1 to enhance affinity and broaden strain cross-reactivity. Proc. Natl. Acad. Sci. USA 913809-3813. - PMC - PubMed
-
- Batista, F. D., and M. S. Neuberger. 1998. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity 8751-759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

