Distinctive features of adult ocular dominance plasticity
- PMID: 18842887
- PMCID: PMC2851628
- DOI: 10.1523/JNEUROSCI.2451-08.2008
Distinctive features of adult ocular dominance plasticity
Abstract
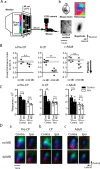
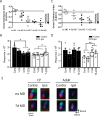
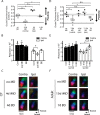
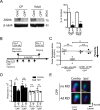
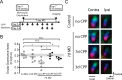
Sensory experience profoundly shapes neural circuitry of juvenile brain. Although the visual cortex of adult rodents retains a capacity for plasticity in response to monocular visual deprivation, the nature of this plasticity and the neural circuit changes that accompany it remain enigmatic. Here, we investigate differences between adult and juvenile ocular dominance plasticity using Fourier optical imaging of intrinsic signals in mouse visual cortex. This comparison reveals that adult plasticity takes longer than in the juvenile mouse, is of smaller magnitude, has a greater contribution from the increase in response to the open eye, and has less effect on the hemisphere ipsilateral to the deprived eye. Binocular deprivation also causes different changes in the adult. Adult plasticity is similar to juvenile plasticity in its dependence on signaling through NMDA receptors. We propose that adult ocular dominance plasticity arises from compensatory mechanisms that counterbalance the loss of afferent activity caused by visual deprivation.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
