The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons
- PMID: 18842893
- PMCID: PMC2572775
- DOI: 10.1523/JNEUROSCI.1917-08.2008
The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons
Abstract
The development of distinct cellular layers and precise synaptic circuits is essential for the formation of well functioning cortical structures in the mammalian brain. The extracellular protein Reelin, through the activation of a core signaling pathway, including the receptors ApoER2 and VLDLR (very low density lipoprotein receptor) and the adapter protein Dab1 (Disabled-1), controls the positioning of radially migrating principal neurons, promotes the extension of dendritic processes in immature forebrain neurons, and affects synaptic transmission. Here we report for the first time that the Reelin signaling pathway promotes the development of postsynaptic structures such as dendritic spines in hippocampal pyramidal neurons. Our data underscore the importance of Reelin as a factor that promotes the maturation of target neuronal populations and the development of excitatory circuits in the postnatal hippocampus. These findings may have implications for understanding the origin of cognitive disorders associated with Reelin deficiency.
Figures
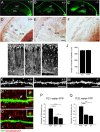
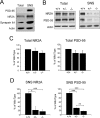
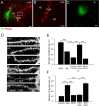
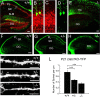

Similar articles
-
Interfering of the Reelin/ApoER2/PSD95 Signaling Axis Reactivates Dendritogenesis of Mature Hippocampal Neurons.J Cell Physiol. 2017 May;232(5):1187-1199. doi: 10.1002/jcp.25605. Epub 2016 Oct 25. J Cell Physiol. 2017. PMID: 27653801
-
Reelin activates SRC family tyrosine kinases in neurons.Curr Biol. 2003 Jan 8;13(1):18-26. doi: 10.1016/s0960-9822(02)01403-3. Curr Biol. 2003. PMID: 12526740
-
Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway.Neuron. 2004 Jan 8;41(1):71-84. doi: 10.1016/s0896-6273(03)00819-5. Neuron. 2004. PMID: 14715136
-
Reelin Functions, Mechanisms of Action and Signaling Pathways During Brain Development and Maturation.Biomolecules. 2020 Jun 26;10(6):964. doi: 10.3390/biom10060964. Biomolecules. 2020. PMID: 32604886 Free PMC article. Review.
-
Nonneuronal roles for the reelin signaling pathway.Dev Dyn. 2017 Apr;246(4):217-226. doi: 10.1002/dvdy.24462. Epub 2016 Nov 17. Dev Dyn. 2017. PMID: 27739126 Review.
Cited by
-
Reelin depletion protects against autoimmune encephalomyelitis by decreasing vascular adhesion of leukocytes.Sci Transl Med. 2020 Aug 12;12(556):eaay7675. doi: 10.1126/scitranslmed.aay7675. Sci Transl Med. 2020. PMID: 32801146 Free PMC article.
-
Selective Inactivation of Reelin in Inhibitory Interneurons Leads to Subtle Changes in the Dentate Gyrus But Leaves Cortical Layering and Behavior Unaffected.Cereb Cortex. 2020 Mar 14;30(3):1688-1707. doi: 10.1093/cercor/bhz196. Cereb Cortex. 2020. PMID: 31667489 Free PMC article.
-
Co-cultures of cerebellar slices from mice with different reelin genetic backgrounds as a model to study cortical lamination.F1000Res. 2023 Oct 2;11:1183. doi: 10.12688/f1000research.126787.2. eCollection 2022. F1000Res. 2023. PMID: 37881513 Free PMC article.
-
Gender-specific effect of Mthfr genotype and neonatal vigabatrin interaction on synaptic proteins in mouse cortex.Neuropsychopharmacology. 2011 Jul;36(8):1714-28. doi: 10.1038/npp.2011.52. Epub 2011 Apr 13. Neuropsychopharmacology. 2011. PMID: 21490592 Free PMC article.
-
Protein-Protein Interaction Among the FoxP Family Members and their Regulation of Two Target Genes, VLDLR and CNTNAP2 in the Zebra Finch Song System.Front Mol Neurosci. 2017 May 1;10:112. doi: 10.3389/fnmol.2017.00112. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28507505 Free PMC article.
References
-
- Arnaud L, Ballif BA, Förster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol. 2003;13:9–17. - PubMed
-
- Ballif BA, Arnaud L, Cooper JA. Tyrosine phosphorylation of Disabled-1 is essential for Reelin-stimulated activation of Akt and Src family kinases. Brain Res Mol Brain Res. 2003;117:152–159. - PubMed
-
- Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, Herz J. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor ApoER2. Neuron. 2005;47:567–579. - PubMed
-
- Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol. 2003;13:18–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
