GABAergic afferents activate both GABAA and GABAB receptors in mouse substantia nigra dopaminergic neurons in vivo
- PMID: 18842898
- PMCID: PMC2757944
- DOI: 10.1523/JNEUROSCI.2387-08.2008
GABAergic afferents activate both GABAA and GABAB receptors in mouse substantia nigra dopaminergic neurons in vivo
Abstract
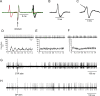
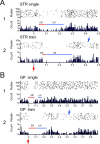
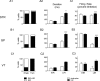
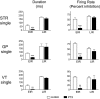
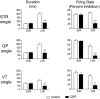
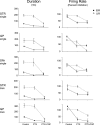
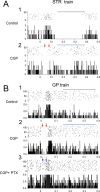
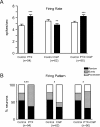
Most in vivo electrophysiological studies of substantia nigra have used rats. With the recent proliferation of the use of mice for in vitro neurophysiological studies because of the availability of various genetically modified strains to identify the roles of various channels and proteins in neuronal function, it is crucial to obtain data on in vivo responses in mice to verify that the in vitro results reflect functioning of systems comparable with those that have been well studied in rat. Inhibitory responses of rat nigral dopaminergic neurons by stimulation of afferents from striatum, globus pallidus, or pars reticulata have been shown to be mediated predominantly or exclusively by GABA(A) receptors. This is puzzling given the substantial expression of GABA(B) receptors and the ubiquitous appearance of GABA(B) synaptic responses in rat dopaminergic neurons in vitro. In the present study, we studied electrically evoked GABAergic inhibition in nigral dopaminergic neurons in C57BL/6J mice. Stimulation of the three major GABAergic inputs elicited stronger and longer-lasting inhibitory responses than those seen in rats. The early inhibition was GABA(A) mediated, whereas the later component, absent in rats, was GABA(B) mediated and selectively enhanced by GABA uptake inhibition. Striatal-evoked inhibition exhibited a slower onset and a weaker initial component compared with inhibition from globus pallidus or substantia nigra pars reticulata. These results are discussed with respect to differences in the size and neuronal density of the rat and mouse brain and the different sites of synaptic contact of the synapses from the three GABAergic afferents.
Figures


References
-
- Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994;265:412–415. - PubMed
-
- Bishop KM, Wahlsten D. Sex and species differences in mouse and rat forebrain commissures depend on the method of adjusting for brain size. Brain Res. 1999;815:358–366. - PubMed
-
- Bolam JP, Smith Y. The GABA and substance P input to dopaminergic neurones in the substantia nigra of the rat. Brain Res. 1990;529:57–78. - PubMed
-
- Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur J Pharmacol. 1994;269:219–224. - PubMed
-
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
