Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats
- PMID: 18842902
- PMCID: PMC2577155
- DOI: 10.1523/JNEUROSCI.1850-08.2008
Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats
Abstract
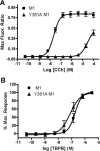
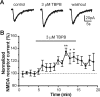
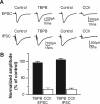
Recent studies suggest that subtype-selective activators of M(1)/M(4) muscarinic acetylcholine receptors (mAChRs) may offer a novel approach for the treatment of psychotic symptoms associated with schizophrenia and Alzheimer's disease. Previously developed muscarinic agonists have provided clinical data in support of this hypothesis, but failed in clinical development because of a lack of true subtype specificity and adverse effects associated with activation of other mAChR subtypes. We now report characterization of a novel highly selective agonist for the M(1) receptor with no agonist activity at any of the other mAChR subtypes, termed TBPB [1-(1'-2-methylbenzyl)-1,4'-bipiperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one]. Mutagenesis and molecular pharmacology studies revealed that TBPB activates M(1) through an allosteric site rather than the orthosteric acetylcholine binding site, which is likely critical for its unprecedented selectivity. Whole-cell patch-clamp recordings demonstrated that activation of M(1) by TBPB potentiates NMDA receptor currents in hippocampal pyramidal cells but does not alter excitatory or inhibitory synaptic transmission, responses thought to be mediated by M(2) and M(4). TBPB was efficacious in models predictive of antipsychotic-like activity in rats at doses that did not produce catalepsy or peripheral adverse effects of other mAChR agonists. Finally, TBPB had effects on the processing of the amyloid precursor protein toward the non-amyloidogenic pathway and decreased Abeta production in vitro. Together, these data suggest that selective activation of M(1) may provide a novel approach for the treatment of symptoms associated with schizophrenia and Alzheimer's disease.
Figures








References
-
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51–58. - PubMed
-
- Ansari MS, Kessler RM, Clanton JA, de Paulis T, Baldwin RM. Comparison of three [18F]fallypride methods intended for automated remote chemistry modules. J Nucl Med. 2006;47:159P.
-
- Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, Shannon HE, Tollefson GD, Rasmussen K, Bymaster FP, Hurley DJ, Potter WZ, Paul SM. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997a;54:465–473. - PubMed
-
- Bodick NC, Offen WW, Shannon HE, Satterwhite J, Lucas R, van Lier R, Paul SM. The selective muscarinic agonist xanomeline improves both the cognitive deficits and behavioral symptoms of Alzheimer disease. Alzheimer Dis Assoc Disord. 1997b;11(Suppl 4):S16–S22. - PubMed
-
- Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
