Effects of odor stimulation on antidromic spikes in olfactory sensory neurons
- PMID: 18842957
- PMCID: PMC2604858
- DOI: 10.1152/jn.90399.2008
Effects of odor stimulation on antidromic spikes in olfactory sensory neurons
Abstract
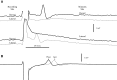
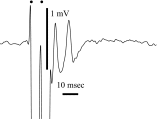
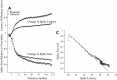
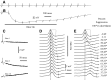
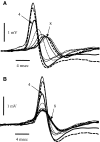
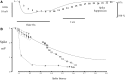
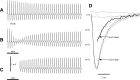
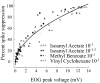
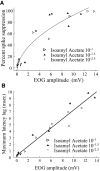
Spikes were evoked in rat olfactory sensory neuron (OSN) populations by electrical stimulation of the olfactory bulb nerve layer in pentobarbital anesthetized rats. The latencies and recording positions for these compound spikes showed that they originated in olfactory epithelium. Dual simultaneous recordings indicated conduction velocities in the C-fiber range, around 0.5 m/s. These spikes are concluded to arise from antidromically activated olfactory sensory neurons. Electrical stimulation at 5 Hz was used to track changes in the size and latency of the antidromic compound population spike during the odor response. Strong odorant stimuli suppressed the spike size and prolonged its latency. The latency was prolonged throughout long odor stimuli, indicating continued activation of olfactory receptor neuron axons. The amounts of spike suppression and latency change were strongly correlated with the electroolfactogram (EOG) peak size evoked at the same site across odorants and across stimulus intensities. We conclude that the curve of antidromic spike suppression gives a reasonable representation of spiking activity in olfactory sensory neurons driven by odorants and that the correlation of peak spike suppression with the peak EOG shows the accuracy of the EOG as an estimate of intracellular potential in the population of olfactory sensory neurons. In addition, these results have important implications about traffic in olfactory nerve bundles. We did not observe multiple peaks corresponding to stimulated and unstimulated receptor neurons. This suggests synchronization of spikes in olfactory nerve, perhaps by ephaptic interactions. The long-lasting effect on spike latency shows that action potentials continue in the nerve throughout the duration of an odor stimulus in spite of many reports of depolarization block in olfactory receptor neuron cell bodies. Finally, strong odor stimulation caused almost complete block of antidromic spikes. This indicates that a very large proportion of olfactory axons was activated by single strong odor stimuli.
Figures



Similar articles
-
Regional distribution of rat electroolfactogram.J Neurophysiol. 1995 Jun;73(6):2207-20. doi: 10.1152/jn.1995.73.6.2207. J Neurophysiol. 1995. PMID: 7666133
-
Discrimination among odorants by single neurons of the rat olfactory bulb.J Neurophysiol. 1989 Jun;61(6):1161-77. doi: 10.1152/jn.1989.61.6.1161. J Neurophysiol. 1989. PMID: 2746317
-
Responses of mitral/tufted cells to orthodromic and antidromic electrical stimulation in the olfactory bulb of the tiger salamander.J Neurophysiol. 1988 Jun;59(6):1736-55. doi: 10.1152/jn.1988.59.6.1736. J Neurophysiol. 1988. PMID: 3404202
-
Spatial patterning of response to odors in the peripheral olfactory system.Physiol Rev. 1976 Jul;56(3):578-93. doi: 10.1152/physrev.1976.56.3.578. Physiol Rev. 1976. PMID: 778869 Review.
-
Coding odor identity and odor value in awake rodents.Prog Brain Res. 2014;208:205-22. doi: 10.1016/B978-0-444-63350-7.00008-5. Prog Brain Res. 2014. PMID: 24767484 Free PMC article. Review.
Cited by
-
Odorant-specific modes of signaling in mammalian olfaction.Chem Senses. 2010 Sep;35(7):533-9. doi: 10.1093/chemse/bjq045. Epub 2010 Jun 2. Chem Senses. 2010. PMID: 20519266 Free PMC article. Review. No abstract available.
References
-
- Astic L, Saucier D, Holley A. Topographical relationships between olfactory receptor cells and glomerular foci in the rat olfactory bulb. Brain Res 424: 144–152, 1987. - PubMed
-
- Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron 20: 69–81, 1998. - PubMed
-
- Bliss TV, Rosenberg ME. Activity-dependent changes in conduction velocity in the olfactory nerve of the tortoise. Pfluegers 381: 209–216, 1979. - PubMed
-
- Brunet LJ, Gold GH, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron 17: 681–693, 1996. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

