The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury
- PMID: 18843253
- PMCID: PMC2652647
- DOI: 10.1038/ki.2008.500
The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury
Abstract
Chemokines and their receptors such as CCR2 and CX3CR1 mediate leukocyte adhesion and migration into injured tissue. To further define mechanisms of monocyte trafficking during kidney injury we identified two groups of F4/80-positive cells (F4/80(low) and F4/80(high)) in the normal mouse kidney that phenotypically correspond to macrophages and dendritic cells, respectively. Following ischemia and 3 h of reperfusion, there was a large influx of F4/80(low) inflamed monocytes, but not dendritic cells, into the kidney. These monocytes produced TNF-alpha, IL-6, IL-1alpha and IL-12. Ischemic injury induced in CCR2(-/-) mice or in CCR2(+/+) mice, made chimeric with CCR2(-/-) bone marrow, resulted in lower plasma creatinine levels and their kidneys had fewer infiltrated F4/80(low) macrophages compared to control mice. CX3CR1 expression contributed to monocyte recruitment into inflamed kidneys, as ischemic injury in CX3CR1(-/-) mice was reduced, with fewer F4/80(low) macrophages than controls. Monocytes transferred from CCR2(+/+) or CX3CR1(+/-) mice migrated into reperfused kidneys better than monocytes from either CCR2(-/-) or CX3CR1(-/-) mice. Adoptive transfer of monocytes from CCR2(+/+) mice, but not CCR2(-/-) mice, reversed the protective effect in CCR2(-/-) mice following ischemia-reperfusion. Egress of CD11b(+)Ly6C(high) monocytes from blood into inflamed kidneys was CCR2- and CX3CR1-dependent. Our study shows that inflamed monocyte migration, through CCR2- and CX3CR1-dependent mechanisms, plays a critical role in kidney injury following ischemia reperfusion.
Figures
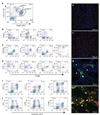
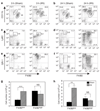


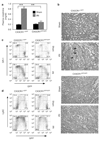
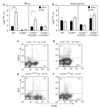

Comment in
-
First responders: understanding monocyte-lineage traffic in the acutely injured kidney.Kidney Int. 2008 Dec;74(12):1509-11. doi: 10.1038/ki.2008.555. Kidney Int. 2008. PMID: 19034300
References
-
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. - PubMed
-
- Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2:356–365. - PubMed
-
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210. - PubMed
-
- Li L, Okusa MD. Blocking the immune response in ischemic acute kidney injury: the role of adenosine 2A agonists. Nat Clin Pract Nephrol. 2006;2:432–444. - PubMed
-
- Jo SK, Sung SA, Cho WY, et al. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant. 2006;21:1231–1239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

