Neuroimaging in dementia
- PMID: 18843575
- PMCID: PMC2647854
- DOI: 10.1055/s-0028-1083695
Neuroimaging in dementia
Abstract
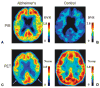
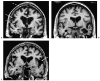
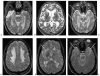
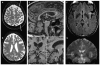
Although dementia is a clinical diagnosis, neuroimaging often is crucial for proper assessment. Magnetic resonance imaging (MRI) and computed tomography (CT) may identify nondegenerative and potentially treatable causes of dementia. Recent neuroimaging advances, such as the Pittsburgh Compound-B (PIB) ligand for positron emission tomography imaging in Alzheimer's disease, will improve our ability to differentiate among the neurodegenerative dementias. High-resolution volumetric MRI has increased the capacity to identify the various forms of the frontotemporal lobar degeneration spectrum and some forms of parkinsonism or cerebellar neurodegenerative disorders, such as corticobasal degeneration, progressive supranuclear palsy, multiple system atrophy, and spinocerebellar ataxias. In many cases, the specific pattern of cortical and subcortical abnormalities on MRI has diagnostic utility. Finally, among the new MRI methods, diffusion-weighted MRI can help in the early diagnosis of Creutzfeldt-Jakob disease. Although only clinical assessment can lead to a diagnosis of dementia, neuroimaging is clearly an invaluable tool for the clinician in the differential diagnosis.
Figures

Similar articles
-
The clinical diagnosis of early-onset dementias: diagnostic accuracy and clinicopathological relationships.Brain. 2011 Sep;134(Pt 9):2478-92. doi: 10.1093/brain/awr189. Epub 2011 Aug 11. Brain. 2011. PMID: 21840888
-
Neuroimaging in dementia.Neurotherapeutics. 2011 Jan;8(1):82-92. doi: 10.1007/s13311-010-0012-2. Neurotherapeutics. 2011. PMID: 21274688 Free PMC article. Review.
-
Neuroimaging in neurodegenerative dementias.Semin Neurol. 2012 Sep;32(4):347-60. doi: 10.1055/s-0032-1331808. Epub 2013 Jan 29. Semin Neurol. 2012. PMID: 23361481 Review.
-
Diffusion tensor magnetic resonance imaging for single subject diagnosis in neurodegenerative diseases.Brain. 2013 Jul;136(Pt 7):2253-61. doi: 10.1093/brain/awt118. Epub 2013 May 31. Brain. 2013. PMID: 23729473
-
Dementia spectrum disorders: lessons learnt from decades with PET research.J Neural Transm (Vienna). 2019 Mar;126(3):233-251. doi: 10.1007/s00702-019-01975-4. Epub 2019 Feb 14. J Neural Transm (Vienna). 2019. PMID: 30762136 Free PMC article. Review.
Cited by
-
Apolipoprotein E-dependent load of white matter hyperintensities in Alzheimer's disease: a voxel-based lesion mapping study.Alzheimers Res Ther. 2015 May 15;7(1):27. doi: 10.1186/s13195-015-0111-8. eCollection 2015. Alzheimers Res Ther. 2015. PMID: 25984242 Free PMC article.
-
Pattern Recognition to Objectively Differentiate the Etiology of Cognitive Decline: Analysis of the Impact of Stroke and Alzheimer's Disease.Neuroepidemiology. 2020;54(6):446-453. doi: 10.1159/000510133. Epub 2020 Oct 5. Neuroepidemiology. 2020. PMID: 33017832 Free PMC article.
-
Pattern Recognition to Identify Stroke in the Cognitive Profile: Secondary Analyses of a Prospective Cohort Study.Cerebrovasc Dis Extra. 2019;9(3):114-122. doi: 10.1159/000503002. Epub 2019 Oct 8. Cerebrovasc Dis Extra. 2019. PMID: 31593944 Free PMC article.
-
Neuroimaging in vascular cognitive impairment: a state-of-the-art review.BMC Med. 2016 Nov 3;14(1):174. doi: 10.1186/s12916-016-0725-0. BMC Med. 2016. PMID: 27806705 Free PMC article. Review.
-
Classification Prediction of Alzheimer's Disease and Vascular Dementia Using Physiological Data and ECD SPECT Images.Diagnostics (Basel). 2024 Feb 7;14(4):365. doi: 10.3390/diagnostics14040365. Diagnostics (Basel). 2024. PMID: 38396404 Free PMC article.
References
-
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
-
- Maurer K, Volk S, Gerbaldo H. Auguste D and Alzheimer’s disease. Lancet. 1997;349:1546–1549. - PubMed
-
- Klunemann HH, Fronhofer W, Wurster H, Fischer W, Ibach B, Klein HE. Alzheimer’s second patient: Johann F and his family. Ann Neurol. 2002;52:520–523. - PubMed
-
- Karas G, Scheltens P, Rombouts S, et al. Precuneus atrophy in early-onset Alzheimer’s disease: a morphometric structural MRI study. Neuroradiology. 2007;49:967–976. - PubMed

