Oestrogens and progestins for preventing and treating postpartum depression
- PMID: 18843619
- PMCID: PMC7061327
- DOI: 10.1002/14651858.CD001690.pub2
Oestrogens and progestins for preventing and treating postpartum depression
Abstract
Background: Postpartum depression is a common complication of childbirth, affecting approximately 13% of women. A hormonal aetiology has long been hypothesised due to the sudden and substantial fluctuations in concentrations of steroid hormones associated with pregnancy and the immediate postpartum period. There is also convincing evidence that oestrogens, progestins, and related compounds have important central nervous system activity at physiological concentrations.
Objectives: The primary objective of this review was to assess the effects of oestrogens and progestins, including natural progesterone and synthetic progestogens, compared with placebo or usual antepartum, intrapartum, or postpartum care in the prevention and treatment of postpartum depression.
Search strategy: We searched The Cochrane Pregnancy and Childbirth Group trials register (June 2004), the Cochrane Depression Anxiety and Neurosis Group trials register (July 2004), the Cochrane Central Register of Controlled Trials (July 2004), MEDLINE (1966 to 2004), EMBASE (1980 to 2004), and CINAHL (1982 to 2004). We scanned secondary references and contacted experts in the field.
Selection criteria: All published and unpublished randomised controlled trials comparing an oestrogen and progestin intervention with a placebo or usual antepartum, intrapartum, or postpartum care among pregnant women or new mothers recruited within the first year postpartum.
Data collection and analysis: Two review authors participated in the evaluation of methodological quality, data extraction, and data analysis. Results are presented using relative risk for categorical data and weighted mean difference for continuous data.
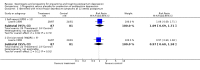
Main results: Two trials, involving 229 women, met the selection criteria. Norethisterone enanthate, a synthetic progestogen, administered within 48 hours of delivery was associated with a significantly higher risk of developing postpartum depression. Oestrogen therapy was associated with a greater improvement in depression scores than placebo among women with severe depression.
Authors' conclusions: Synthetic progestogens should be used with significant caution in the postpartum period. The role of natural progesterone in the prevention and treatment of postpartum depression has yet to be evaluated in a randomised, placebo-controlled trial. Oestrogen therapy may be of modest value for the treatment of severe postpartum depression. Its role in the prevention of recurrent postpartum depression has not been rigorously evaluated. Further research is warranted.
Conflict of interest statement
Lori Ross is currently a co‐investigator of an ongoing trial that is evaluating the effect of transdermal estradiol patches in women with postpartum depression.
Figures










Update of
-
Oestrogens and progestogens for preventing and treating postnatal depression.Cochrane Database Syst Rev. 2000;(2):CD001690. doi: 10.1002/14651858.CD001690. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2008 Oct 08;(4):CD001690. doi: 10.1002/14651858.CD001690.pub2. PMID: 10796270 Updated.
References
References to studies included in this review
Gregoire 1996 {published data only}
-
- Gregoire AJP, Kumar R, Everitt B, Henderson AF, Studd JWW. Transdermal oestrogen for the treatment of severe postnatal depression. Lancet 1996;347:930‐3. - PubMed
-
- Gregoire AJP, Kumar R, Everitt B, Henderson AF, Studd JWW. Transdermal oestrogen for treatment of severe postnatal depression. International Journal of Gynecology & Obstetrics 1996;55:88. - PubMed
-
- Henderson A, Studd JWW, Gregoire A, Kumar R. The role of oestrogen in the treatment of post‐natal depression. Proceedings of 26th British Congress of Obstetrics and Gynaecology;1992; Manchester, UK. 1992:36.
-
- Henderson AF, Gregoire AJP, Kumar RC, Studd JWW. Treatment of severe postnatal depression with oestradiol skin patches. Lancet 1991;338:816‐7. - PubMed
Lawrie 1998 {published data only}
-
- Lawrie T, Hofmeyr G, Jager M, Berk M. A double blind randomised placebo controlled trial of norethisterone enantate: effect on postnatal depression. XXIst Collegium Internationale Neuro‐psychopharmacologicum; 1998 July 12‐16; Glasgow, Scotland. 1998. - PubMed
-
- Lawrie T, Hofmeyr G, Jager M, Berk M. The effect of norethisterone enantate on postnatal depression: a randomised controlled trial. Proceedings of the 17th Conference on Priorities in Perinatal Care; 1998; South Africa. 1998:68.
-
- Lawrie TA, Hofmeyr GJ, Jager M, Berk M. The effect of norethisterone enanthate on postnatal depression: a randomised placebo‐controlled trial. Women's Health Issues 1998;8:199‐200. - PubMed
-
- Lawrie TA, Hofmeyr GJ, Jager M, Berk M, Paiker J, Viljoen E. A double‐blind randomised placebo controlled trial of postnatal norethisterone enanthate: the effect on postnatal depression and serum hormones. British Journal of Obstetrics and Gynaecology 1998;105:1082‐90. - PubMed
References to studies excluded from this review
Ahokas 1998 {published data only}
-
- Ahokas AJ, Turtiainen S, Aito M. Sublingual oestrogen treatment of postnatal depression. Lancet 1998;351(9096):109. - PubMed
Ahokas 1999a {published data only}
-
- Ahokas A, Kaukoranta J, Aito M. Effect of oestradiol on postpartum depression. Psychopharmacology 1999;146(1):108‐10. - PubMed
Ahokas 1999b {published data only}
-
- Ahokas A, Aito M. Role of estradiol in puerperal psychosis. Psychopharmacology 1999;147:108‐10. - PubMed
Ahokas 2000 {published data only}
-
- Ahokas A, Aito M, Rimon R. Positive treatment effect of estradiol in postpartum psychosis: a pilot study. Journal of Clinical Psychiatry 2000;61(3):166‐9. - PubMed
Ahokas 2001 {published data only}
-
- Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta‐estradiol: a preliminary study. Journal of Clinical Psychiatry 2001;62(5):332‐6. - PubMed
Ball 1999 {published data only}
-
- Ball DE, Morrison P. Oestrogen transdermal patches for post partum depression in lactating mothers ‐ a case report. Central African Journal of Medicine 1999;45:68‐70. - PubMed
Cizza 1997 {published data only}
-
- Cizza G, Gold P, Chrousos G. High‐dose transdermal estrogen, corticotropin‐releasing hormone, and postnatal depression. Journal of Clinical Endocrinology and Metabolism 1997;82:704. - PubMed
Dalton 1985 {published data only}
-
- Dalton K. Progesterone prophylaxis used successfully in postnatal depression. Practitioner 1985;229:507‐8.
Dalton 1989 {published data only}
-
- Dalton K. Sucessful prophylactic progesterone for idiopathic postnatal depression. International Journal of Prenatal and Perinatal Studies 1989;1:322‐7.
Kumar 2003 {published data only}
-
- Kumar C, McIvor R, Davies T, Brown N, Papadopoulos A, Wieck A, Checkley S, Campbell I, Marks M. Estrogen adminisration does not reduce the rate of recurrence of affective psychosis after childbirth. Journal of Clinical Psychiatry 2003;64:112‐8. - PubMed
Sichel 1995 {published data only}
-
- Sichel D, Cohen L, Robertson L, Ruttenberg A, Rosenbaum J. Prophylactic estrogen in recurrent postpartum affective disorder. Biological Psychiatry 1995;38:814‐8. - PubMed
Van Der Meer 1984 {published data only}
-
- Meer YG, Loendersloot EW, Loenen AC. Effect of high‐dose progesterone in postpartum depression. Journal of Psychosomatic Obstetrics and Gynaecology 1984;3:67‐8.
References to ongoing studies
NIMH 2003 {published data only}
-
- National Institue of Mental Health (NIMH). Clinical trial of estrogen for postpartum depression. ClinicalTrials.gov (http://clinicaltrials.gov/ct2/show/NCT00059228) (accessed 15 September 2004) 2003.
Steiner 2004 {unpublished data only}
-
- Steiner M. Randomized, double‐blind, placebo‐controlled trial of low‐dose transdermal 17‐estradiol acceleration of sertraline in major depression with postpartum onset. Personal communication 2004.
Additional references
Albrecht 1990
-
- Albrecht ED, Pepe GJ. Placental steroid hormone biosynthesis in primate pregnancy. Endocrine reviews 1990;11(1):124‐50. - PubMed
Bell 1994
-
- Bell AJ, Land NM, Milne S, Hassanyeh F. Long‐term outcome of post‐partum psychiatric illness requiring admission. Journal of Affective Disorders 1994;31(1):67‐70. - PubMed
Bloch 2000
-
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. American Journal of Psychiatry 2000;157(6):924‐30. - PubMed
Bloch 2003
-
- Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Comprehensive Psychiatry 2003;44(3):234‐46. - PubMed
Essex 2001
-
- Essex MJ, Klein MH, Miech R, Smider NA. Timing of initial exposure to maternal major depression and children's mental health symptoms in kindergarten. British Journal of Psychiatry 2001;179:151‐6. - PubMed
Galea 2001
-
- Galea LA, Wide JK, Barr AM. Estradiol alleviates depressive‐like symptoms in a novel animal model of post‐partum depression. Behavioural Brain Research 2001;122(1):1‐9. - PubMed
Hay 2001
-
- Hay DF, Pawlby S, Sharp D, Asten P, Mills A, Kumar R. Intellectual problems shown by 11‐year‐old children whose mothers had postnatal depression. Journal of Child Psychology & Psychiatry & Allied Disciplines 2001;42(7):871‐89. - PubMed
Murray 1999
-
- Murray L, Sinclair D, Cooper P, Ducournau P, Turner P, Stein A. The socioemotional development of 5‐year‐old children of postnatally depressed mothers. Journal of Child Psychology & Psychiatry & Allied Disciplines 1999;40:1259‐71. - PubMed
Murray 2001
-
- Murray L, Woolgar M, Cooper P, Hipwell A. Cognitive vulnerability to depression in 5‐year‐old children of depressed mothers. Journal of Child Psychology and Psychiatry 2001;42:891‐9. - PubMed
Nott 1976
-
- Nott PN, Franklin M, Armitage C, Gelder MG. Hormonal changes and mood in the puerperium. British Journal of Psychiatry 1976;128:379‐83. - PubMed
O'Hara 1996
-
- O'Hara MW, Swain AM. Rates and risk of postpartum depression‐a meta‐analysis. International Review of Psychiatry 1996;8(1):37‐54.
RevMan 2004 [Computer program]
-
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
Rosendaal 2003
-
- Rosendaal FR, Hylckama Vlieg A, Tanis BC, Helmerhorst FM. Estrogens, progestogens and thrombosis. Journal of Thrombosis and Haemostasis 2003;1:1371‐1380. - PubMed
Ross 2004
-
- Ross LE, Sellers EM, Gilber Evans SE, Romach MK. Mood changes during pregnancy and the postpartum period: development of a biopsychosocial model. Acta Psychiatrica Scandinavica 2004;109:457‐66. - PubMed
Russell 2001
-
- Russell JA, Douglas AJ, Ingram CD. Brain preparations for maternity‐‐adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation. An overview. Progress in Brain Research 2001;133:1‐38. - PubMed
Soares 2003
-
- Soares CN, Poitras JR, Prouty J. Effect of reproductive hormones and selective estrogen receptor modulators on mood during menopause. Drugs & Aging 2003;20(2):85‐100. - PubMed
Steiner 2003
-
- Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. Journal of Affective Disorders 2003;74(1):67‐83. - PubMed
Stern 1983
-
- Stern G, Kruckman L. Multi‐disciplinary perspectives on post‐partum depression: an anthropological critique. Social Science & Medicine 1983;17(15):1027‐41. - PubMed
Stuart 1998
-
- Stuart S, Couser G, Schilder K, O'Hara M, Gorman L. Postpartum anxiety and depression: onset and comorbidity in a community sample. Journal of Nervous & Mental Disease 1998;186:420‐4. - PubMed
Sugawara 1999
-
- Sugawara M, Sakamoto S, Kitamura T, Toda MA, Shima S. Structure of depressive symptoms in pregnancy and the postpartum period. Journal of Affective Disorders 1999;54(1‐2):161‐9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

