Granulopoiesis-stimulating factors to prevent adverse effects in the treatment of malignant lymphoma
- PMID: 18843642
- PMCID: PMC7144686
- DOI: 10.1002/14651858.CD003189.pub4
Granulopoiesis-stimulating factors to prevent adverse effects in the treatment of malignant lymphoma
Abstract
Background: Granulopoiesis-stimulating factors, such as granulocyte-colony-stimulating factor (G-CSF) and granulocyte-macrophage-colony-stimulating factor (GM-CSF), are being used to prevent febrile neutropenia and infection in patients undergoing treatment for malignant lymphoma. The question of whether G-CSF and GM-CSF improve dose intensity, tumour response, and overall survival in this patient population has not been answered yet. Since the results from single studies are inconclusive, a systematic review was undertaken.
Objectives: To determine the effectiveness of G-CSF and GM-CSF in patients with malignant lymphoma with respect to preventing neutropenia, febrile neutropenia and infection; improving quality of life, adherence to treatment protocol, tumour response, freedom from treatment failure (FFTF) and overall survival (OS); and adverse effects.
Search strategy: We searched The Cochrane Library, MEDLINE, EMBASE, CancerLit, and other relevant literature databases; Internet databases of ongoing trials; and conference proceedings of the American Society of Clinical Oncology and the American Society of Hematology (1980 - 2007). We included full-text and abstract publications as well as unpublished data.
Selection criteria: Randomised controlled trials comparing prophylaxis with G-CSF or GM-CSF versus placebo/no prophylaxis in adult patients with malignant lymphoma undergoing chemotherapy were included for review. Both study arms had to receive identical chemotherapy and supportive care.
Data collection and analysis: Trial eligibility and quality assessment, data extraction and analysis were done by two reviewers independently. Authors were contacted to obtain missing data.
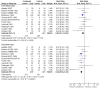
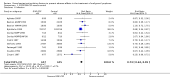
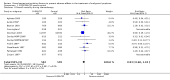
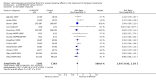
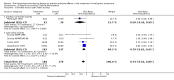
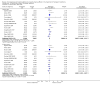
Main results: We included 13 eligible randomised controlled trials with 2607 randomised patients. Compared with no prophylaxis, both G-CSF and GM-CSF did not improve overall survival (hazard ratio 0.97; 95% CI 0.87 to 1.09) or FFTF (hazard ratio 1.11; 95% CI 0.91 to 1.35). Prophylaxis significantly reduced the relative risk (RR) for severe neutropenia (RR 0.67; 95% confidence interval (CI) 0.60 to 0.73), febrile neutropenia (RR 0.74; 95% CI 0.62 to 0.89) and infection (RR 0.74; 95% CI 0.64 to 0.85). There was no evidence that either G-CSF or GM-CSF reduced the number of patients requiring intravenous antibiotics (RR 0.82; 95%CI 0.57 to 1.18); lowered infection related mortality (RR 0.93; 95% CI 0.51 to 1.71); or improved complete tumour response (RR 1.03; 95% CI 0.95 to 1.10).One study evaluated quality of life parameters and found no differences between the treatment groups.
Authors' conclusions: G-CSF and GM-CSF, when used as a prophylaxis in patients with malignant lymphoma undergoing conventional chemotherapy, reduce the risk of neutropenia, febrile neutropenia and infection. However, based on the randomised trials currently available, there is no evidence that either G-CSF or GM-CSF provide a significant advantage in terms of complete tumour response, FFTF or OS.
Conflict of interest statement
Chugai Pharma (Chugai Pharma Marketing Ltd., Subsidiary Germany, Frankfurt/Main) provided the translation of a Japanese publication (Togawa 2000) for the Cochrane Haematological Malignancies Group. Andreas Engert received research funding and honoraria from Amgen Ltd. for other projects.
Figures









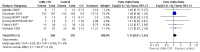
















































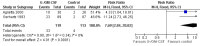


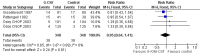

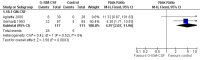




























































Update of
-
Granulopoiesis-stimulating factors to prevent adverse effects in the treatment of malignant lymphoma.Cochrane Database Syst Rev. 2004;(3):CD003189. doi: 10.1002/14651858.CD003189.pub3. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2008 Oct 08;(4):CD003189. doi: 10.1002/14651858.CD003189.pub4. PMID: 15266474 Updated.
References
References to studies included in this review
Aglietta 2000 {published data only}
-
- Aglietta M, Montemurro F, Fagioli F, Volta C, Botto B, Cantonetti M, et al. Short term treatment with Escherichia coli recombinant human granulocyte‐macrophage‐colony stimulating factor prior to chemotherapy for Hodgkin disease. Cancer 2000;88:454‐60. - PubMed
Aglietta 2000* {unpublished data only}
-
- Personal communication in addition to Aglietta 2000.
Avilés 1994 {published data only}
-
- Avilés A, Díaz‐Maqueo JC, Talavera A, Nambo MJ, García EL. Effect of granulocyte colony‐stimulating factor in patients with diffuse large cell lymphoma treated with intensive chemotherapy. Leukemia and Lymphoma 1994;15:153‐7. - PubMed
Avilés 1994* {unpublished data only}
-
- Personal communication in addition to Avilès 1994.
Bastion 1993 {published data only}
-
- Bastion Y, Bosly A, Gisselbrecht C, Reyes F, Tilly H, Herbrecht R, et al. A randomized double‐blind phase III study of Filgrastim (recombinant human G‐CSF) vs placebo during intensive induction chemotherapy in 55 to 59 year old patients (pts) with poor prognosis aggressive Non‐Hodgkin's Lymphoma. Blood. 1993; Vol. 82 Suppl (1):143a.
Bastion ACVBP 1993 {published data only}
-
- Data on patients from the Bastion 1993 study, who received ACVBP chemotherapy.
Bastion VIMMM 1993 {published data only}
-
- Data on patients from the Bastion 1993 study, who received VIMMM chemotherapy.
Björkholm 1999 {published and unpublished data}
-
- Björkholm M, Osby E, Hagberg H, Kvaloy S, Teerenhovi L, Myhre J, et al. Randomized trial of r‐metHu granulocyte colony‐stimulating factor (G‐CSF) as adjunct to CHOP or CNOP treatment of elderly patients with aggressive non‐Hodgkin's lymphoma. Blood. 1999; Vol. 94, 10 Suppl (1):599a.
Björkholm CHOP 1999 {published and unpublished data}
-
- Data on patients from the Björkholm 1999 study, who received CHOP chemotherapy.
Björkholm CNOP 1999 {published and unpublished data}
-
- Data on patients from the Björkholm 1999 study, who received CNOP chemotherapy.
Burton 2006 {published data only}
Burton CHOP 2006 {published data only}
-
- Data on patients from the Burton 2006 study who received CHOP.
Burton PMitCEBO 2006 {published data only}
-
- Data from patients in the Burton 2006 study who received PMitCEBO.
Cunningham* {unpublished data only}
-
- Cunningham D. Randomised trial of platinum based chemotherapy +/‐G‐CSF in relapsed Non‐Hodgkins and Hodgkins Lymphoma.
Doorduijn 2003 {published data only}
-
- Doorduijn JK, an der Holt B, Imhoff GW, Hem KG, Kramer MHH, Oers MHJ, et al. CHOP compared to CHOP plus granulocyte colony‐stimulating factor in elderly patients with aggressive Non‐Hodgkin's Lymphoma. Journal of Clinical Oncology 2003;21(16):3041‐50. - PubMed
Dunlop 1998 {published data only}
-
- Dunlop DJ, Eatock MM, Paul J, Anderson S, Reed NS, Soukop M, et al. Randomized multicentre trial of filgrastim as an adjunct to combination chemotherapy for Hodgkin's disease. West of Scotland Lymphoma Group. Clinical Oncology (Royal College of Radiologists) 1998;10:107‐14. - PubMed
Dunlop MOPP 1998 {published data only}
-
- Data on patients from the Dunlop 1998 study, who received MOPP chemotherapy.
Dunlop MOPP 1998* {unpublished data only}
-
- Personal communication in addition to Dunlop MOPP 1994.
Dunlop MOPP/EVAP 98 {published data only}
-
- Data on patients from the Dunlop 1998 study, who received MOPP/EVAP chemotherapy.
Dunlop MOPP/EVAP 98* {unpublished data only}
-
- Personal communication in addition to Dunlop MOPP/EVAP 1998.
Engelhard 1994 {published data only}
-
- Engelhard M, Gerhartz H, Brittinger G, Engert A, Fuchs R, Geiseler B, et al. Cytokine efficiency in the treatment of high‐grade malignant non‐ Hodgkin's lymphomas: Results of a randomized double‐blind placebo‐ controlled study with intensified COP‐BLAM plus‐or‐minus sign rhGM‐ CSF.. Annals of Oncology 1994;5:123‐5. - PubMed
Fridrik 1997 {published data only}
-
- Fridrik MA, Greil R, Hausmaninger H, Krieger O, Oppitz P, Stoger M, et al. Randomized open label phase III trial of CEOP/IMVP‐Dexa alternating chemotherapy and filgrastim versus CEOP/IMVP‐Dexa alternating chemotherapy for aggressive non‐Hodgkin's lymphoma (NHL). A multicenter trial by the Austrian Working Group for Medical Tumor Therapy. Annals of Hematology 1997;75:135‐40. - PubMed
Fridrik 1997* {unpublished data only}
-
- Personal communication in addition to Fridrik 1997.
Gerhartz 1993 {published data only}
-
- Gerhartz HH, Engelhard M, Meusers P, Brittinger G, Wilmanns W, Schlimock G, et al. Randomized, double‐blind, placebo‐controlled, phase III study of recombinant human granulocyte‐macrophage colony‐stimulating factor as adjunct to induction treatment of high‐grade malignant Non‐Hodgkin's Lymphoma. Blood 1993;82(8):2329‐39. - PubMed
Gerhartz 1994a {published data only}
-
- Gerhartz HH, Engelhard M, Brittinger G, Schlimok G, Thiel E, Huber C, et al. Recombinant human granulocyte‐macrophage colony‐stimulating factor as adjunct to chemotherapy in aggressive non‐Hodgkin's lymphomas. Seminars in Oncology 1994;21, 6 Suppl (16): 25‐8. - PubMed
Gisselbrecht 1997 {published data only}
-
- Gisselbrecht C, Haioun C, Lepage E, Bastion Y, Tilly H, Bosly A, et al. Placebo‐controlled phase III study of Lenograstim (glycosylated recombinant human granulocyte colony‐stimulating factor) in aggressive non‐Hodgkin's lymphoma: factors influencing chemotherapy administration. Groupe d'Etude des Lymphomes de l'Adulte. Leukemia and Lymphoma 1997;25:289‐300. - PubMed
Gisselbrecht 1997* {unpublished data only}
-
- Personal communication in addition to Gisselbrecht 1997.
Pettengell 1992 {published data only}
-
- Pettengell R, Gurney H, Radford JA, Deakin DP, James R, Wilkinson PM, et al. Granulocyte colony‐stimulating factor to prevent dose‐limiting neutropenia in non‐Hodgkin's lymphoma: a randomized controlled trial. Blood 1992;80(6):1430‐6. - PubMed
Souêtre 1994 {published data only}
-
- Souêtre E, Qing W. Economic analysis of Lenograstim in the correction of neutropenia following chemotherapy for non‐Hodgkin's lymphoma. PharmacoEconomics 1994;6, Suppl (2):36‐43. - PubMed
Zinzani 1997 {published data only}
-
- Zinzani PL, Pavone E, Storti S, Moretti L, Fattori PP, Guardigni L, et al. Randomized trial with or without granulocyte colony‐stimulating factor as adjunct to induction VNCOP‐B treatment of elderly high‐grade non‐Hodgkin's lymphoma. Blood 1997;89(11):3974‐9. - PubMed
Zinzani 1997* {unpublished data only}
-
- Personal communication in addition to Zinzani 1997.
Zinzani 1999 {published data only}
-
- Zinzani PL, Storti S, Zaccaria A, Moretti L, Magagnoli M, Pavone E, et al. Elderly aggressive‐histology non‐Hodgkin's lymphoma: first‐line VNCOP‐B regimen experience on 350 patients. Blood 1999;94(1):33‐8. - PubMed
Ösby 2003 {published data only}
-
- Ösby K, Hagberg H, Kvaloy S, Teerenhovi L, Anderson H, Cavallin‐Stahl E, et al. CHOP is superior to CNOP in elderly patients with aggressive lymphoma while outcome is unaffected by filgrastim treatment: results of a Nordic Lymphoma Group randomized trial. Blood 2003;101(10):3840‐8. - PubMed
References to studies excluded from this review
Adde 1998 {published data only}
-
- Adde M, Shad A, Venzon D, Arndt C, Gootenberg J, Neely J, et al. Additional chemotherapy agents improve treatment outcome for children and adults with advanced B‐cell lymphoma. Seminars in Oncology 1998;25, 2 suppl (4):33‐9. - PubMed
Anaissie 1996 {published data only}
-
- Anaissie EJ, Vartivarian S, Bodey GP, Legrand C, Kantarjian H, Abi‐Said D, et al. Randomized comparison between antibiotics alone and antibiotics plus granulocyte‐macrophage colony‐stimulating factor (Escherichia coli‐derived) in cancer patients with fever and neutropenia. The American Journal of Medicine 1996;100:17‐23. - PubMed
Bergmann 1995 {published data only}
-
- Bergmann L, Karakas T, Knuth A, Lautenschlager G, Mitrou PS, Hoelzer D. Recombinant human granulocyte‐macrophage colony‐stimulating factor after combined chemotherapy in high‐grade non‐Hodgkin's lymphoma ‐ a randomised pilot study. European Journal of Cancer 1995;31A:2164‐8. - PubMed
Bertini 1996 {published data only}
-
- Bertini M, Freilone R, Vitolo U, Botto B, Ciotti R, Cinieri S, et al. The treatment of elderly patients with aggressive non‐Hodgkin's lymphomas: feasibility and efficacy of an intensive multidrug regimen.. Leukemia and Lymphoma 1996;22:483‐93. - PubMed
Bodey 1994 {published data only}
-
- Bodey GP, Anaissie E, Gutterman J, Vadhan‐Raj S. Role of granulocyte‐macrophage colony‐stimulating factor as adjuvant treatment in neutropenic patients with bacterial and fungal infection. European journal of clinical microbiology & infectious diseases 1994;13 Suppl (2):18‐22. - PubMed
Gerhartz 1993ex {published data only}
-
- Gerhartz HH, Stern AC, Wolf‐Hornung B, Kazempour M, Schmetzer H, Gugerli U, et al. Intervention treatment of established neutropenia with human recombinant granulocyte‐macrophage colony‐stimulating factor (rhGM‐CSF) in patients undergoing cancer chemotherapy. Leukemia Research 1993;17(2):175‐85. - PubMed
Gianni 1990 {published data only}
-
- Gianni AM, Bregni M, Siena S, Orazi A, Stern AC, Gandola L, et al. Recombinant human granulocyte‐macrophage colony‐stimulating factor reduces hematologic toxicity and widens clinical applicability of high‐dose cyclophosphamide treatment in breast cancer and non‐Hodgkin's lymphoma. Jounral of Clinical Oncology 1990;8(5):768‐78. - PubMed
Gordon 1999 {published data only}
-
- Gordon LI, Young M, Weller E, Habermann TM, Winter JN, Glick J, et al. A phase II trial of 200% ProMACE‐CytaBOM in patients with previously untreated aggressive lymphomas: Analysis of response, toxicity, and dose intensity. Blood 1999;94(10):3307‐14. - PubMed
Gregory 1998 {published data only}
-
- Gregory S, Goh YT, Fuerst T, O'Brien T, Giles FJ. Fludarabine, cyclophosphamide and GM‐CSF is effective in chronic lymphocytic leukemia and low grade non Hodgkins lymphoma. Blood. 1998; Vol. 92 Suppl (10):276b.
Gustavsson 1997 {published data only}
-
- Gustavsson A. G‐CSF (filgrastim) as an adjunct to MOPP/ABVD therapy in Hodgkin's disease. Acta Oncologica 1997;36(5):483‐8. - PubMed
Hansen 1995 {published data only}
-
- Hansen PB, Johnsen HE, Ralfkiaer E, Jensen L, Gaarsdal E, Hansen NE. Short‐term rhG‐CSF priming before chemotherapy does mobilize blood progenitors but does not prevent chemotherapy induced myelotoxicity: a randomized study of patients with non‐Hodgkin's lymphomas. Leukemia and Lymphoma 1995;19:453‐60. - PubMed
Hartmann 1997 {published data only}
-
- Hartmann LC, Tschetter LK, Habermann TM, Ebbert LP, Johnson PS, Mailliard JA, et al. Granulocyte colony‐stimulating factor in severe chemotherapy‐induced afebrile neutropenia. New England Journal of Medicine 1997;336(25):1776‐80. - PubMed
Ho 1990 {published data only}
-
- Ho AD, Del VF, Haas R, Engelhard M, Hiddemann W, Ruckle H, et al. Sequential studies on the role of mitoxantrone, high‐dose cytarabine, and recombinant human granulocyte‐macrophage colony‐stimulating factor in the treatment of refractory non‐Hodgkin's lymphoma. Seminars in Oncology 1990;17:14‐9. - PubMed
Hovgaard 1992 {published data only}
-
- Hovgaard DJ, Nissen NI. Effect of recombinant human granulocyte‐macrophage colony‐stimulating factor in patients with Hodgkin's disease: A Phase I/II Study. Journal of Clinical Oncology 1992;10(3):390‐7. - PubMed
Kaku 1993 {published data only}
-
- Kaku K, Takahashi M, Moriyama Y, Nakahata T, Masaoka T, Yoshida Y, et al. Recombinant human granulocyte‐macrophage colony‐stimulating factor (rhGM‐CSF) after chemotherapy in patients with non‐Hodgkin's lymphoma; a placebo‐controlled double blind phase III trial. Leukemia and Lymphoma 1993;11(3‐4):229‐38. - PubMed
Kaneko 1991 {published data only}
-
- Kaneko T, Takaku F, Ogawa M. Outline of clinical studies on recombinant human granulocyte colony stimulating factor (KRN 8601) in Japan. Tokai Journal of Experimental & Clinical Medicine 1991;16(1):51‐61. - PubMed
Kaplan 1991 {published data only}
-
- Kaplan LD, Kahn JO, Crowe S, Northfelt D, Neville P, Grossberg H, et al. Clinical and virologic effects of recombinant human granulocyte‐macrophage colony‐stimulating factor in patients receiving chemotherapy for human immunodeficiency virus‐associated non‐Hodgkin's lymphoma: results of a randomized trial. Journal of Clinical Oncology 1991;9(6):929‐40. - PubMed
Karthaus 1998 {published data only}
-
- Karthaus M, Rosenthal C, Huebner G, Paul H, Elser C, Hertenstein B, et al. Effect of topical oral G‐CSF on oral mucositis: a randomised placebo‐controlled trial. Bone Marrow Transplantation 1998;22(8):781‐5. - PubMed
Liberati 1991 {published data only}
-
- Liberati AM, Cinieri S, Schippa M, Clemente F, Filippo S, Grignani F. GM‐CSF: clinical trials in non‐Hodgkin's lymphoma patients with chemotherapy induced leucopenia. Leukemia 1991;5 Suppl (1): 119‐22. - PubMed
Lopez‐Hernandez 2000 {published data only}
-
- Lopez‐Hernandez MA, Jimenez‐Alvarado R, Borbolla‐Escoboza R, Flores‐Chapa JD, Alvarado‐Ibarra M, Gonzalez‐Avante M, et al. Factor estimulante de colonias de granulocitos en el tratamiento de neutropenia febril. Gaceta medica de Mexico 2000;136(2):99‐105. - PubMed
Magrath 1996 {published data only}
-
- Magrath I, Adde M, Shad A, Venzon D, Seibel N, Gootenberg J, et al. Adults and children with small non‐cleaved‐cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. Journal of Clinical Oncology 1996;14(3):925‐34. - PubMed
Maher 1994 {published data only}
-
- Maher DW, Lieschke GJ, Green M, Bishop J, Stuart‐Harris R, Wolf M, et al. Filgrastim in patients with chemotherapy‐induced febrile neutropenia. American College of Physicians 1994;121:492‐501. - PubMed
Maiche 1993 {published data only}
-
- Maiche AG, Muhonen T. Granulocyte colony‐stimulating factor (G‐CSF) with or without a quinolone in the prevention of infection in cancer patients. European Journal of Cancer 1993;29A(10):1403‐5. - PubMed
Mangiagalli 1995 {published data only}
-
- Mangiagalli M, Miccolis I, Maffe P, Pogliani EM, Corneo G. Role of granulocyte colony‐stimulating factor in relapsed/resistant intermediate and high‐grade non‐Hodgkin's lymphoma patients treated with the E‐SHAP regimen. Tumori 1995;81:91‐5. - PubMed
Mayordomo 1995 {published data only}
-
- Mayordomo JI, Rivera F, Díaz‐Puente MT, Lianes P, Colomer R, López‐Brea M, et al. Improving treatment of chemotherapy‐induced neutropenic fever by administration of colony‐stimulating factors. Journal of the National Cancer Institute 1995;87(11):803‐8. - PubMed
Moreau 1997 {published data only}
-
- Moreau P, Fiere D, Bezwoda WR, Facon T, Attal M, Laporte JP, et al. Prospective randomized placebo‐controlled study of granulocyte‐macrophage colony‐stimulating factor without stem‐cell transplantation after high‐dose melphalan in patients with multiple myeloma. Journal of Clinical Oncology 1997;15(2):660‐6. - PubMed
Motoyoshi 1986 {published data only}
-
- Motoyoshi K, Takaku F, Maekawa T, Miura Y, Kimura K, Furusawa S, et al. Protective effect of partially purified human urinary colony‐stimulating factor on granulocytopenia after antitumor chemotherapy. Experimental Hematology 1986;14:1069‐75. - PubMed
Niitsu 1995 {published data only}
-
- Niitsu N, Umeda M. Usefulness of COP‐BLAM Therapy with concomitant G‐CSF in elderly patients with non‐Hodgkin's lymphoma in comparison with patients not given G‐CSF. Japanese Journal of Geriatrics 1995;32(6):410‐5. - PubMed
Ogawa 1990 {published data only}
-
- Ogawa M, Masaoka T, Mizoguchi H, Takaku F, Nakashima M. [A phase III study of KRN 8601 (rhG‐CSF) on neutropenia induced by chemotherapy for malignant lymphoma‐‐a multi‐institutional placebo controlled double‐blind comparative study]. Gan‐To‐Kagaku‐Ryoho. Cancer & chemotherapy 1990;17(3):365‐73. - PubMed
Riccardi 1993 {published data only}
-
- Riccardi A, Gobbi P, Danova M, Giordano M, Pieresca C, Bertoloni D, et al. MOPP/ABVD/CAD chemotherapy with and without recombinant human granulocyte‐macrophage colony stimulating factor in untreated, unfavorable prognosis Hodgkin's disease. Haematologica 1993;78(1):44‐8. - PubMed
Seymour 1995 {published data only}
-
- Seymour AM, Campos E, Thatcher N, Greve J, Cunningham D, Howell A, et al. A single‐blind, randomised, vehicle‐controlled dose‐finding study of recombinant human granulocyte colony‐stimulating factor (lenograstim) in patients undergoing chemotherapy for solid cancers and lymphoma. European Journal of Cancer 1995;31A:2157‐63. - PubMed
Shi 1994 {published data only}
-
- Shi YK, Sun Y, Su M. Clinical study of recombinant human granulocyte‐macrophage colony‐stimulating factor on chemotherapy‐induced leukopenia. Chung Hua Chung Liu Tsa Chih 1994;16(5):356‐9. - PubMed
Shi 1996 {published data only}
-
- Shi YK, Feng FY, Liu H‐B. Clinical study of recombinant human granulocyte colony‐stimulating factor (rhG‐CSF) on leukopenia induced by chemotherapy with CHOP and CAF regimen in cancer patients. Chinese Journal of Clinical Oncology 1996;23(4):252‐6. - PubMed
Togawa 2000 {published data only}
-
- Togawa A, Mizoguchi H, Toyama K, Urabe A, Ohasi Y, Takaku F. Clinical evalutaion of rhG‐CSF in patients with neutropenia induced by chemotherapy for multiple myeloma. Rinsho Ketsueki. The Japanese Journal of Clinical Hematology 2000;41(2):115‐22. - PubMed
Unpublished trial {unpublished data only}
-
- Phase III randomized double‐blind study of intensive chemotherapy with ARA‐C/CACP/VP‐16 plus GM‐CSF vs placebo in patients with relapsing or refractory intermediate‐ and high‐grade non‐Hodgkin's lymphoma (summary last modified 11/90). http://cancernet.nci.nih.gov/cgi‐bin/ 28.12.2000; Vol. Protocol IDs: AECM‐8903071, NCI‐V89‐0161, UW‐C89‐049‐05, NCI‐V89‐0252, MAOP‐3189, NCI‐V90‐0118, MSKCC‐90085, NCI‐V90‐0148.
Vellenga 1996 {published data only}
-
- Vellenga E, Uyl‐de‐Groot CA, Wit R, Keizer HJ. Löwenberg B, Haaft MA, et al. Randomized placebo‐controlled trial of granulocyte‐macrophage colony‐stimulating factor in patients with chemotherapy‐related febrile neutropenia. Journal of Clinical Oncology 1996;14(2):619‐27. - PubMed
Wilson 1998 {published data only}
-
- Wilson WH, Little R, Pearson D, Jaffe ES, Steinberg SM, Cheson BD, et al. Phase II and dose‐escalation with or without granulocyte colony‐stimulating factor study of 9‐aminocamptothecin in relapsed and refractory lymphomas. Journal of Clinical Oncology 1998;16(7):2345‐51. - PubMed
Yau 1996 {published data only}
-
- Yau JC, Neidhart JA, Triozzi P, Verma S, Nemunaitis J, Quick DP, et al. Randomized placebo‐controlled trial of granulocyte‐macrophage colony‐stimulating‐factor support for dose‐intensive cyclophosphamide, etoposide, and cisplatin. American Journal of Hematology 1996;51:289‐95. - PubMed
Yoshida 1999 {published data only}
-
- Yoshida M, Karasawa M, Naruse T, Fukuda M, Hirashima K, Oh H, et al. Effect of granulocyte‐colony stimulating factor on empiric therapy with flomoxef sodium and tobramycin in febrile neutropenic patients with hematological malignancies. Kan‐etsu Hematological Disease and Infection Study Group.. International Journal of Hematology 1999;69:81‐8. - PubMed
Zagonel 1994 {published data only}
-
- Zagonel V, Babare R, Merola MC, Talamini R, Lazzarini R, Tirelle U, et al. Cost‐benefit of granulocyte colony‐stimulating factor administration in older patients with non‐Hodgkin's lymphoma treated with combination chemotherapy. Annals of Oncology 1994;5 Suppl (2):S127‐32. - PubMed
References to ongoing studies
Blay {unpublished data only}
-
- Blay JY. Elypse 2. personal communication.
Additional references
Alvarado Ibarra 1999
-
- Alvarado Ibarra ML, Borbolla Escoboza JR, López‐Hernández MA, González‐Avante CM, Flores Chapa JDD, Trueba Christy E, et al. Neutrophil recovery time and adverse side effects in acute leukemia patients treated with intensive chemotherapy and concomitant G or GM‐CSF. La Revista de Investigación Clínica 1999;51:77‐80. - PubMed
Armitage 1993
-
- Armitage JO. The treatment of non‐Hodgkin's lymphoma. New England Journal of Medicine 1993;328(14):1023‐30. - PubMed
ASCO Guidelines 1994
-
- No authors listet. American Society of Clinical Oncology Recommendations for the Use of Hematopoietic Colony‐Stimulating Factors: Evidence‐Based, Clinical Practice Guidelines. Journal of Clinical Oncology 1994;12(11):2471‐508. - PubMed
ASCO Guidelines 1996
-
- No authors listet. Update of Recommendations for the Use of Hematopoietic Colony‐Stimulating Factors: Evidence‐Based Clinical Practice Guidelines. Journal of Clinical Oncology 1996;14(6):1957‐60. - PubMed
ASCO Guidelines 2000
-
- Ozer H, Armitage JO, Bennett CL, Crawford J, Demetri GD, Pizzo PA, et al. 2000 Update of recommendations for the use of hematopoietic colony‐stimulating factors: evidence‐based, clinical practice guidelines. Journal of Clinical Oncology 2000;18(20):3558‐85. - PubMed
ASCO Guidelines 2006
-
- Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence‐based clinical practice guideline. J Clin Oncol 2006;24(19):3187‐205. - PubMed
Barbui 1996
-
- Barbui T, Finazzi G, Grassi A, Marchioli R. Thrombosis in cancer patients treated with hematopoietic growth factors ‐ a meta‐analysis. On behalf of the Subcommittee on Haemaostasis and Malignancy of the Scientific and Standardization Committee of the ISTH. Thrombosis and Haemostasis 1996;75(2):368‐71. - PubMed
Bennett 2000a
-
- Bennett CL, Stinson TJ, Bhoopalam N, Marriott M, Panganiban J, Kozloff MF. A double‐Blind, randomized trial of toxicity, resource use and costs for filgrastim and sargramostim. Conference Proceedings of the American Society of Hematology. 2000:abstract no 1712.
Bennett 2000b
-
- Bennett CL, Stinson TJ, Laver JH, Bishop MR, Godwin JE, Tallman MS. Cost analysis of adjunct colony stimulating factors for acute leukemia: can they improve clinical decision making. Leukemia and Lymphoma 2000;37(1‐2):65‐70. - PubMed
Beveridge 1998
-
- Beveridge RA, Miller JA, Kales AN, Binder RA, Robert NJ, Harvey JH, et al. A comparison of efficacy of sargramostim (yeast‐derived rhuGM‐CSF) and filgrastim (bacteria‐derived rhuG‐CSF) in the therapeutic setting of chemotherapy‐induced myelosuppression. Cancer Investigation 1998;16(6):366‐73. - PubMed
Bobey 1998
-
- Bobey N, Woodman RC. Neutropenic complications in advanced‐stage non‐Hodgkin lymphoma: implications for the use of prophylactic recombinant human granulocyte‐colony stimulating factor (G‐CSF). Clinical and Investigative Medicine ‐ Medicine Clinique et Experimentale 1998;21(2):63‐70. - PubMed
Bodey 1966
-
- Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Annals of Internal Medicine 1966;64(2):328‐40. - PubMed
Bodey 1986
-
- Bodey GP. Infection in cancer patients. The American Journal of Medicine 1986;81 Suppl (1A): 11‐26. - PubMed
Bow 1998
-
- Bow EJ. Infection risk and cancer chemotherapy: the impact of the chemotherapeutic regimen in patients with lymphoma and solid tissue malignancies. Journal of Antimicrobial Chemotherapy 1998;41 Suppl (D):1‐5. - PubMed
Bronchud 1988
Bui 1995
-
- Bui BN, Chevallier B, Chevreau C, Krakowski I, Peny AM, Thyss A, et al. Efficacy of lenograstim on hematologic tolerance to MAID chemotherapy in patients with advanced soft tissue sarcoma and consequences on treatment dose‐intensity. Journal of Clinical Oncology 1995;13(10):2629‐36. - PubMed
Chevallier 1995
-
- Chevallier B, Chollet P, Merrouche Y, Roche H, Fumoleau P, Kerbat P, et al. Lenograstim prevents morbidity from intensive induction chemotherapy in the treatment of inflammatory breast cancer. Journal of Clinical Oncology 1995;13(7):1564‐71. - PubMed
Crawford 1991
-
- Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, et al. Reduction by granulocyte colony‐stimulating factor of fever and neutropenia induced by chemotherapy in patients with small‐cell lung‐cancer. New England Journal of Medicine 1991;325(3):164‐70. - PubMed
de Graaf 1996
-
- Graaf H, Willemse PH, Bong SB, Piersma H, Tjabbes T, Veelen H, et al. Dose intensity of standard adjuvant CMF with granulocyte colony‐stimulating factor for premenopausal patients with node‐positive breast cancer. Oncology 1996;53(4):289‐94. - PubMed
de Witte 1992
-
- Witte T, Gratwohl A, LN, Bacigalupo A, Stern AC, Speck B, et al. Recombinant human granulocyte‐macrophage colony‐stimulating factor accelerates neutrophil and monocyte recovery after allogeneic T‐cell‐depleted bone marrow transplantation. Blood 1992;79(5):1359‐65. - PubMed
Deb 1998
-
- Deb G, Donfrancesco A, Slo LD, Cozza R, Castellano A, Paole F, et al. Shortened time to recovery from chemotherapy induced neutropenia in pediatric patients with high dose combined cytokines. Anticancer Research 1998;18(1B):489‐92. - PubMed
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn M. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. Systematic Reviews in Health Care. Meta‐analysis in context. 2nd Edition. London: BMJ Books, 2001:285‐312.
Dempke 2000
-
- Dempke W, Poblozki A, Grothey A, Schmoll HJ. Human hematopoietic growth factors: Old lessons and new perspectives. Anticancer Research 2000;20:5155‐64. - PubMed
DeVita 1987
-
- DeVita VT, Hubbard SM, Longo DL. The chemotherapy of lymphomas: looking back, moving forward. The Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Research 1987;47(22):5810‐24. - PubMed
Dickersin 1994
Diehl 2003
-
- Diehl V, Franklin J, Pfreundschuh M, Lathan B, Paulus U, Hasenclever D, et al. Standard and increased‐dose BEACOPP chemotherapy compared with COPP‐ABVD for advanced Hodgkin's disease. New England Journal of Medicine 2003;348(24):2386‐95. - PubMed
Egger 1997
Engert 1994
-
- Engert A, Schaadt M, Diehl V. Malignant Lymphoma [Maligne Lymphome]. In: Classen M, Diehl V, Kochsiek K editor(s). Innere Medizin. 3rd Edition. Vol. 1, München ‐ Wien ‐ Baltimore: Urban & Schwarzenberg, 1994:180‐203.
EORTC Guidelines 2006
-
- Aapro MS, Cameron DA, Pettengell R, Bohlius J, Crawford J, Ellis M, et al. EORTC guidelines for the use of granulocyte‐colony stimulating factor to reduce the incidence of chemotherapy‐induced febrile neutropenia in adult patients with lymphomas and solid tumours. European Journal of Cancer 2006;42(15):2433‐53. - PubMed
Freedman 1999
-
- Freedman A, Nadler L. Maligne Diseases of lymphatic cells [Maligne Erkrankungen lymphatischer Zellen]. In: Fauci A, Braunwald E, Isselbacher K, et al. editor(s). Harrisons Innere Medizin. Vol. 1, London: McGraw‐Hill International, 1999:833‐862.
Fukuoka 1997
Goldie 1983
-
- Goldie JH, Coldman AJ. Quantitative model for multiple levels of drug resistance in clinical tumours. Cancer Treatment Reports 1983;67(10):923‐31. - PubMed
Hackshaw 2004
Herrmann 1998
-
- Herrmann R, Drings P. Lymphoproliferative diseases [Lymphoproliferative Erkrankungen]. In: Schettler G, Greten H editor(s). Innere Medizin. 9th Edition. Vol. 1, Stuttgart ‐ New York: Thieme, 1998:1009‐28.
Hryniuk 1984
-
- Hryniuk W, Bush H. The importance of dose intensity in chemotherapy of metastatic breast cancer. Journal of Clinical Oncology 1984;2(11):1281‐8. - PubMed
Hryniuk 1986
-
- Hryniuk W, Levine MN. Analysis of dose intensity for adjuvant chemotherapy trials in stage II breast cancer. Journal of Clinical Oncology 1986;4(8):1162‐70. - PubMed
Hryniuk 1987
-
- Hryniuk WM. Average relative dose intensity and the impact on design of clinical trials. Seminars in Oncology 1987;14(1):65‐74. - PubMed
ICH 1999
-
- ICH Harmonised Tripartite Guideline. Statistical principles for clinical trials. International Conference on Harmonisation E9 Expert Working Group. Statistics in Medicine 1999;18:1905‐42. - PubMed
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Klastersky 2000
-
- Klastersky J, Paesmans M, Rubenstein EB, Boyer M, Elting L, Feld R, et al. The multinational association for supportive care in cancer risk index: A multinational scoring system for identifying low‐risk febrile neutropenic cancer patients. Journal of Clinical Oncology 2000;18(16):3038‐51. - PubMed
Kuderer 2007
-
- Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony‐stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. [Review] [81 refs]. Journal of Clinical Oncology 2007;25(21):3158‐67. - PubMed
Lepage 1993
-
- Lepage E, Gisselbrecht C, Haioun C, Sebban C, Tilly H, Bosley A, et al. Prognostic significance of received relative dose intensity in non‐Hodgkin's lymphoma patients: Application to LNH‐87 protocol. Annals of Oncology 1993;4(8):651‐6. - PubMed
Lopez 1986
-
- Lopez AF, Williamson DJ, Gamble JR, Begley CG, Harlan JM, Klebanoff SJ, et al. Recombinant human granulocyte‐macrophage colony stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surfae receptor expression, and survival. Journal of Clinical Investigation 1986;78(5):1220‐8. - PMC - PubMed
Lydaki 1995
-
- Lydaki E, Bolonaki E, Stiakaki E, Dimitriou H, Kalmantis T, Kalmanti M. Efficacy of recombinant human granulocyte colony‐stimulating factor and recombinant human granuloyte‐macrophage colony‐stimulating factor in neutropenic children with malignancies. Pediatric Hematology and Oncology 1995;12(6):551‐8. - PubMed
Lyman 1995
-
- Lyman GH, Balducci L. A cost analysis of hematopoietic colony‐stimulating factors. Oncology (Huntingt) 1995;9 Suppl (11):85‐91. - PubMed
Lyman 1998
-
- Lyman GH, Kuderer NM, Balducci L. Economic impact of granulopoiesis stimulating agents on the management of febrile neutropenia. Current Opinion in Oncology 1998;10:291‐6. - PubMed
Lyman 2000
-
- Lyman GH. A predictive model for neutropenia associated with cancer chemotherapy. Pharmacotherapy 2000;20(7 Pt 2):104‐11S. - PubMed
Lyman 2002
-
- Lyman GH, Kuderer N, Djulbegovic B. Prophylactic granulocyte colony‐stimulating factor in patients receiving dose‐intensive cancer chemotherapy: A meta‐analysis. The American Journal of Medicine 2002;112:406‐11. - PubMed
Magrath 1997
-
- Magrath I, Adde M, Shad A, Venzon D, Seibel N, Neeley J, et al. Relative efficacy of G‐and GM‐CSF in ameliorating toxicity in an intensive treatment protocol for advanced, diffuse B cell NHLs, predominantly small noncleaved cell lymphomas. Abstracts of the American Society of Hematology. 1997:abstract no 847.
Morstyn 1988
-
- Morstyn G, Campbell L, Souza LM, Alton NK, Keech J, Green M, et al. Effect of granulocyte colony stimulating factor on neutropenia induced by cytotoxic chemotherapy. Lancet 1988;26(1):667‐72. - PubMed
Parmar 1998
-
- Parma MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. - PubMed
Pettengell 1993
-
- Pettengell R, Testa NG, Swindell R, Crowther D, Dexter TM. Transplantation potential of hematopoietic cells released into the circulation during routine chemotherapy for non‐Hodgkin's lymphoma [see comments].. Blood 1993;82:2239‐48. - PubMed
Pinto 2007
-
- Pinto L, Liu Z, Doan Q, Bernal M, Dubois R, Lyman G. Comparison of pegfilgrastim with filgrastim on febrile neutropenia, grade IV neutropenia and bone pain: a meta‐analysis of randomized controlled trials. Current Medical Research & Opinion 2007;23(9):2283‐95. - PubMed
Preundschuh 2004a
-
- Pfreundschuh M, Truemper L, Kloess M, Schmits R, Feller AC, Ruebe C, Rudolph C, Reiser M, Hossfeld DK, Eimermacher H, Hasenclever D, Schmitz N, Loeffler M, German High‐Grade Non‐Hodgkin´s Lymphoma Study Group. Two‐weekly or 3‐weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL‐B2 trial of the DSHNHL. Blood 2004;104(3):634‐641. - PubMed
Preundschuh 2004b
-
- Pfreundschuh M, Truemper L, Kloess M, Schmits R, Feller AC, Rudolph C, Reiser M, Hossfeld DK, Metzner B, Hasenclever D, Schmitz N, Glass B, Ruebe C, Loeffler M, German High‐Grade Non‐Hodgkin´s Lymphoma Study Group. Two‐weekly or 3‐weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good‐prognosis (normal LDH) aggressive lymphomas: results of the NHL‐B1 trial of the DSHNHL. Blood 2004;104(3):626‐633. - PubMed
Rao 2005
-
- Rao R, Shammo JM, Enschede SH, Porter C, Adler SS, Venugopal P, et al. The combination of fludarabine, cyclophosphamide, and granulocyte‐macrophage colony‐stimulating factor in the treatment of patients with relapsed chronic lymphocytic leukemia and low‐grade Non‐Hodgkin's lymphoma. Clinical Lymphoma 2005;6(1):26‐30. - PubMed
Roskos 1998
-
- Roskos LK, Cheung EN, Vincent M, Foote MA. Pharmacology of filgrastim (r‐metHuG‐CSF). In: Morstyn G, Dexter TM, Foote M editor(s). Filgastim (r‐metHuG‐CSF) in Clinical Practice. 2. New York, Basel, Hong Kong: Marcel Dekker, 1998:51‐71.
Rusthoven 1998
-
- Rusthoven J, Bramwell V, Stephenson B. Use of granulocyte colony‐stimulating factor (G‐CSF) in patients receiving myelosuppressive chemotherapy for the treatment of cancer. Cancer Prevention and Control 1998;2(4):179‐90. - PubMed
Skipper 1990
-
- Skipper HE. Dose intensity versus total dose of chemotherapy: an experimental basis. Important Advances in Oncology 1990;1(1):43‐64. - PubMed
Sung 2007
-
- Sung L, Nathan PC, Alibhai SM, Tomlinson GA, Beyene J. Meta‐analysis: effect of prophylactic hematopoietic colony‐stimulating factors on mortality and outcomes of infection. Ann Intern Med 2007;147(6):400‐11. - PubMed
Talcott 1992
-
- Talcott JA, Siegel RD, Finberg R, Goldman L. Risk assessment in cancer patients with fever and neutropenia: a prospective, two‐center validation of a prediction rule. Journal of Clinical Oncology 1992;10(2):316‐22. - PubMed
Timmer‐Bonte 2005
-
- Timmer‐Bonte JN, Boo TM, Smit HJ, Biesma B, Wilschut FA, Cheragwandi SA, et al. Prevention of chemotherapy‐induced febrile neutropenia by prophylactic antibiotics plus or minus granulocyte colony‐stimulating factor in small‐cell lung cancer: a Dutch Randomized Phase III Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2005;23(31):7974‐84. - PubMed
Trillet‐Lenoir 1993
-
- Trillet‐Lenoir V, Green J, Manegold C, Pawel J, Gatzemeier U, Lebeau B, et al. Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. European Journal of Cancer 1993;29A(3):319‐24. - PubMed
Verhagen 1998
-
- Verhagen AP, DeVet HCW, DeBie RA, Kessels AGH, Boers M, Bouter LM, et al. The delphi list: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by delphi consensus. Journal of Clinical Epidemiology 1998;51(12):1235‐41. - PubMed
Vogel 2005
-
- Vogel C, Rader M, Tyulandin S, Wiens B, Neumann T, Carroll R. Pegfilgrastim nearly abrogates occurrence of neutropenic events early in the course of chemotherapy: Results of a phase III, randomized, double‐blind, placebo‐controlled study of patients with breast cancer receiving docetaxel. The Journal of Supportive Oncology 2005;3(2 SUPPL. 1):58‐9.
Woll 1995
-
- Woll PJ, Hodgetts J, Lomax L, Bildet F, Cour‐Chabernaud V, Thatcher N. Can cytotoxic dose‐intensity be increased by using granulocyte colony‐stimulating factor? A randomized controlled trial of lenograstim in small‐cell lung cancer. Journal of Clinical Oncology 1995;13(3):652‐9. - PubMed
Yoshida 1990
-
- Yoshida T, Nakamura S, Ohtake S, Okafuji K, Kobayashi K, Kondo K, et al. Effect of granulocyte colony‐stimulating factor on neutropenia due to chemotherapy for non‐Hodgkin's lymphoma. Cancer 1990;66(9):1904‐9. - PubMed
References to other published versions of this review
Bohlius 2003
-
- Bohlius J, Reiser M, Schwarzer G, Engert A. Impact of granulocyte colony‐stimulating factor (CSF) and granulocyte‐macrophage CSF in patients with malignant lymphoma: a systematic review. British Journal of Haematology 2003;122:413‐23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

