Combination injectable contraceptives for contraception
- PMID: 18843662
- PMCID: PMC6513542
- DOI: 10.1002/14651858.CD004568.pub3
Combination injectable contraceptives for contraception
Update in
-
Combination injectable contraceptives for contraception.Cochrane Database Syst Rev. 2013;3:CD004568. Cochrane Database Syst Rev. 2013. PMID: 23641480
Abstract
Background: Combination injectable contraceptives provide a highly effective, reversible method of preventing pregnancy, and they do not require daily administration or use at the time of coitus. Although they are used in many countries, their acceptability could be limited by method characteristics, such as the need to obtain a monthly injection or bleeding pattern changes.
Objectives: To assess the contraceptive efficacy, bleeding patterns, discontinuation, user preferences, and side effects of combination injectable contraceptives.
Search strategy: We searched computerized databases for randomized controlled trials of combination injectable contraceptives.
Selection criteria: Randomized controlled trials were eligible if they compared a combination injectable with any other contraceptive method (e.g., a second combination injectable contraceptive, progestin-only injectable contraceptive, other hormonal contraceptive or barrier method) or placebo. We limited the review to currently marketed combination injectable contraceptives.
Data collection and analysis: One author evaluated all titles and abstracts from the literature searches to determine their eligibility. Two authors independently extracted data from the eligible trials. Data on contraceptive efficacy, bleeding patterns, continuation, and side effects were entered and analyzed with RevMan.
Main results: Combination injectable contraceptives include depot medroxyprogesterone acetate (DMPA) 25 mg plus estradiol cypionate (E(2)C) 5 mg, as well as norethisterone enanthate (NET-EN) 50 mg plus estradiol valerate (E(2)V) 5 mg. These contraceptives resulted in lower rates of early study discontinuation due to amenorrhea or other bleeding problems than progestin-only contraceptives. However, rates were higher for overall discontinuation and discontinuation due to other medical reasons. Acceptability results favored the combination injectable in one study and the progestin-only in another.Studies comparing two combination injectable contraceptives found that NET-EN 50 mg plus E(2)V 5 mg resulted in less overall discontinuation and less discontinuation due to amenorrhea or prolonged bleeding than DMPA 25 mg plus E(2)C 5 mg. However, these differences were not detected in all trials. The NET-EN plus E(2)V group also had more regular bleeding and fewer prolonged bleeding reference periods than the DMPA plus E(2)C group. The groups did not differ in their amenorrhea rates.
Authors' conclusions: While discontinuation rates can be viewed as a measure of method acceptability, the findings should be interpreted with caution since discontinuation depends on many factors. Future research should be directed toward interventions to improve the acceptability of combination injectable contraceptives, such as providing injections in settings more convenient than clinics, methods for women to administer their own injections, and counseling about possible bleeding pattern changes.
Conflict of interest statement
DA Grimes has consulted with the pharmaceutical companies Bayer Healthcare Pharmaceuticals and Merck & Co, Inc.
Figures






































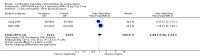
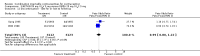


































References
References to studies included in this review
Cuong 1996 {published data only}
-
- Cuong DT, My Huong NT. Comparative phase III clinical trial of two injectable contraceptive preparations, depot‐medroxyprogesterone acetate and Cyclofem, in Vietnamese women. Contraception 1996;54(3):169‐79. - PubMed
Hassan 1999 {published data only}
-
- Hassan EO, el‐Nahal N, el‐Hussein M. Acceptability of the once‐a‐month injectable contraceptives Cyclofem and Mesigyna in Egypt. Contraception 1994;49(5):469‐88. - PubMed
-
- Hassan EO, el‐Nahal N, el‐Hussinie M. Once‐a‐month injectable contraceptives, Cyclofem and Mesigyna, in Egypt. Efficacy, causes of discontinuation, and side effects. Contraception 1999;60(2):87‐92. - PubMed
Indian Council 1990 {published data only}
-
- Datey S, Gaur LN, Saxena BN. Vaginal bleeding patterns of women using different contraceptive methods (implants, injectables, IUDs, oral pills)‐‐an Indian experience. An ICMR Task Force Study. Indian Council of Medical Research. Contraception 1995;51(3):155‐65. - PubMed
-
- Indian Council of Medical Research Task Force on Hormonal Contraception. A multicentre phase III comparative study of two hormonal contraceptive preparations NET‐OEN (50 mg) + E2 valerate (5 mg) given every month and NET‐OEN (200 mg) given every 2 months as intramuscular injection‐‐a report of 12‐month study. Contraception 1990;42(2):179‐90. - PubMed
Mostafa 1994a {published data only}
-
- Mostafa SAM, Sayed GH, Abdel‐Hamid A, Abdullah SA, Saddeek OB, Newton JR, et al. Clinical evaluation of two once‐a‐month injectable contraceptives; Cyclofem and Mesigyna. Assiut Medical Journal 1994;18(4):41‐9.
Piya‐Anant 1998 {published data only}
-
- Piya‐Anant M, Koetsawang S, Patrasupapong N, Dinchuen P, d'Arcangues C, Piaggio G, et al. Effectiveness of Cyclofem in the treatment of depot medroxyprogesterone acetate induced amenorrhea. Contraception 1998;57(1):23‐8. - PubMed
Recio 1986 {published data only}
-
- Recio R, Garza‐Flores J, Schiavon R, Reyes A, Diaz‐Sanchez V, Valles V, et al. Pharmacodynamic assessment of dihydroxyprogesterone acetophenide plus estradiol enanthate as a monthly injectable contraceptive. Contraception 1986;33(6):579‐89. - PubMed
Ruminjo 2005 {published data only}
-
- Ruminjo JK, Sekadde‐Kigondu CB, Karanja JG, Rivera R, Nasution M, Nutley T. Comparative acceptability of combined and progestin‐only injectable contraceptives in Kenya. Contraception 2005;72(2):138‐45. - PubMed
Sang 1995 {published data only}
-
- Sang GW, Shao QX, Ge RS, Ge JL, Chen JK, Song S, et al. A multicentred phase III comparative clinical trial of Mesigyna, Cyclofem and Injectable No. 1 given by intramuscular injection to Chinese women. I. Contraceptive efficacy and side effects. Contraception 1995;51(3):167‐83. - PubMed
-
- Sang GW, Shao QX, Ge RS, Ge JL, Chen JK, Song S, et al. A multicentred phase III comparative clinical trial of Mesigyna, Cyclofem and Injectable No. 1 given by intramuscular injection to Chinese women. II. The comparison of bleeding patterns. Contraception 1995;51(3):185‐92. - PubMed
Simbar 2007 {published and unpublished data}
-
- Simbar M, Tehrani FR, Hashemi Z, Zham H, Fraser IS. A comparative study of Cyclofem® and depot medroxyprogesterone acetate (DMPA) effects on endometrial vasculature. Journal of Family Planning and Reproductive Health Care 2007;33(4):271‐6. - PubMed
Von Kesseru 2000 {published data only}
-
- Kesseru E, Etchepareborda JJ, Wikinski R, Beier S. Premenopause contraception with monthly injectable Mesigyna with special emphasis on serum lipid and bone density patterns. Contraception 2000;61(5):317‐22. - PubMed
WHO 1988 {published data only}
-
- Gómez Alzugaray M, Romeo Gallardo J, Hernández ML, Mojena ML. Efficiency of two injectable contraceptives (cycloprovera and HRP‐102) monthly administered by intramuscular via. Revista Cubana de Obstetricia y Ginecología 1989;15(1‐2):45‐53.
-
- Kazi AI. Comparative evaluation of two once‐a‐month contraceptive injections. Journal of the Pakistan Medical Association 1989;39(4):98‐102. - PubMed
-
- Lang Prieto J, Casas Fernández JA, Pérez García M, Reina Hernández A, Bustillo Tur C. Comparative study of 2 injectable contraceptives administered in monthly doses: cycloprovera and HRP‐102. Revista Cubana de Obstetricia y Ginecología 1993;19(1):53‐63.
-
- Santoso SSI, Affandi B. Efficacy, acceptability and side‐effects of two once‐a‐month injectable contraceptives [Abstract]. Advances in Contraception 1990;6(4):264.
-
- World Health Organization Task Force on Long‐Acting Systemic Agents for Fertility Regulation. A multicentred phase III comparative study of two hormonal contraceptive preparations given once‐a‐month by intramuscular injection. II. The comparison of bleeding patterns. Contraception 1989;40(5):531‐51. - PubMed
WHO 1997 {published data only}
-
- United Nations Development Programme/United Nations Population Fund/World Health Organization/World Bank, Special Programme of Research, Development and Research Training in Human Reproduction. Comparative study of the effects of two once‐a‐month injectable steroidal contraceptives (Mesigyna and Cyclofem) on lipid and lipoprotein metabolism. Contraception 1997;56(4):193‐207. - PubMed
-
- United Nations Development Programme/United Population Fund/World Health Organization/World Bank, Special Programme of Research, Development and Research Training in Human Reproduction, Task Force on Long‐acting Systemic Agents for Fertility Regulation. Comparative study of the effects of two once‐a‐month injectable steroidal contraceptives (Mesigyna® and Cyclofem®) on glucose metabolism and liver function. Contraception 1998;57(2):71‐81. - PubMed
References to studies excluded from this review
Baweja 1985 {published data only}
-
- Baweja R, Bhattacharya SK, Choudhury SD, Krishna U, Manuel M, Phillips FS, et al. Indian Council of Medical Research. Task Force on Hormonal Contraception. Phase II randomized clinical trial with norethisterone oenanthate 50 mg alone and in combination with 5 mg or 2.5 mg of either estradiol valerate or cypionate as a monthly injectable contraceptive. Contraception 1985;32(4):383‐94. - PubMed
Benagiano 1997 {published data only}
-
- Benagiano G, Bianchi P. Carbohydrate metabolism comparative study with the new injectable mesigyna and a three‐phasic oral contraceptive [Abstract]. Advances in Contraception 1995;11(1):39.
-
- Benagiano G, Primiero FM, Bastianelli C, Bianchi P, Medda E. Comparative clinical evaluation of the effect on carbohydrate and lipid metabolism of two norethisterone‐containing hormonal contraceptives: Mesigyna and TriNovum. Contraception 1997;55(5):295‐300. - PubMed
Brucker 2001 {published data only}
-
- Brucker C. Controlled trial with a monthly combination injectable contraceptive in Europe. Gynecological Endocrinology 2001;15(Suppl 3):11‐4. - PubMed
Coutinho 1997 {published data only}
-
- Coutinho EM, Spinola P, Barbosa I, Gatto M, Tomaz G, Morais K, et al. Multicenter, double‐blind, comparative clinical study on the efficacy and acceptability of a monthly injectable contraceptive combination of 150 mg dihydroxyprogesterone acetophenide and 10 mg estradiol enanthate compared to a monthly injectable contraceptive combination of 90 mg dihydroxyprogesterone acetophenide and 6 mg estradiol enanthate. Contraception 1997;55(3):175‐81. - PubMed
Garza‐Flores 1987 {published data only}
-
- Garza‐Flores J, Rodriguez V, Perez‐Palacios G, Virutamasen P, Tang‐Keow P, Konsayreepong R, et al. A multicentered pharmacokinetic, pharmacodynamic study of once‐a‐month injectable contraceptives. I. Different doses of HRP112 and of DepoProvera. World Health Organization Task Force on Long‐acting Systemic Agents for Fertility Regulation. Contraception 1987;36(4):441‐57. - PubMed
Jain 2000 {published data only}
-
- Jain JK, Ota F, Mishell DR Jr. Comparison of ovarian follicular activity during treatment with a monthly injectable contraceptive and a low‐dose oral contraceptive. Contraception 2000;61(3):195‐8. - PubMed
Karim 1971 {published data only}
-
- Karim M, el‐Mahgoub S. Conception control by cyclic injections of norethisterone enanthate and estradiol unducelate. American Journal of Obstetrics and Gynecology 1971;110(5):740‐2. - PubMed
Kashanian 2013 {published data only}
-
- Kashanian M. A comparison between the side effects of low dose combined contraceptive pills and Cyclofem. http://apps.who.int/trialsearch/trial.aspx?trialid=IRCT138903242624N6 (accessed 01 Jan 2013).
Kesseru 1988 {published data only}
-
- Kesseru E, Tozzini R. Monthly injectable contraception with norethisterone enanthate plus estradiol valerate. In: Runnebaum B, Rabe T, Kiesel L editor(s). Female contraception: update and trends. Berlin: Springer‐Verlag, 1988:221‐6.
Khalaf 1994 {published data only}
-
- Khalaf AA, Mostafa SA, Sayed GH, Saddeek OB, Abdullah SA. Monthly injectable contraceptives: are they a valuable addition to the birth control cafeteria [abstract]. British Journal of Obstetrics and Gynaecology 1994;101(3):269‐74.
Meng 1990 {published data only}
-
- Meng YX, Jiang HY, Chen AJ, Lu FY, Yang H, Zhang MY, et al. Hemostatic changes in women using a monthly injectable contraceptive for one year. Contraception 1990;42(4):455‐66. - PubMed
Mostafa 1994b {published data only}
-
- Mostafa SAM, Abdel‐Aleem H, Sayed GH, Abdullah SA, Saddeek OB, Newton JR, et al. Changes in serum lipids, lipoproteins and apolipoproteins during the use of two once‐a‐month injectable contraceptives; Cyclofem and Mesigyna. Assiut Medical Journal 1994;18(4):33‐9.
Werawatgoompa 1980 {published data only}
-
- Werawatgoompa S, Vaivanijkul B, Leepipatpaiboon S, Channiyom K, Virutamasen P, Dusitsin N. The effect of injectable norethisterone oenanthate on ovarian hormones in Thai women. Contraception 1980;21(3):299‐309. - PubMed
WHO 2003 {published data only}
-
- United Nations Development Programme/United Nations Population Fund/World Health Organization/World Bank Special Programme of Research, Development and Research Training in Human Reproduction, Task Force on Long‐acting Systemic Agents for Fertility Regulation. Comparative study of the effects of two once‐a‐month injectable contraceptives (Cyclofem and Mesigyna) and one oral contraceptive (Ortho‐Novum 1/35) on coagulation and fibrinolysis. Contraception 2003;68(3):159‐76. - PubMed
Additional references
Bahamondes 1997a
-
- Bahamondes L, Lavin P, Ojeda G, Petta C, Diaz J, Maradiegue E, et al. Return of fertility after discontinuation of the once‐a‐month injectable contraceptive Cyclofem. Contraception 1997;55(5):307‐10. - PubMed
Bahamondes 1997b
-
- Bahamondes L, Marchi NM, Nakagava HM, Melo ML, Cristofoletti MdeL, Pellini E, et al. Self‐administration with Uniject of the once‐a‐month injectable contraceptive Cyclofem. Contraception 1997;56(5):301‐4. - PubMed
Bassol 1994
-
- Bassol S, Garza‐Flores J. Review of ovulation return upon discontinuation of once‐a‐month injectable contraceptives. Contraception 1994;49(5):441‐53. - PubMed
Belsey 1986
-
- Belsey EM, Machin D, d'Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction. Contraception 1986;34(3):253‐60. - PubMed
Glasier 2003
-
- Glasier AF, Smith KB, Spuy ZM, Ho PC, Cheng L, Dada K, et al. Amenorrhea associated with contraception‐an international study on acceptability. Contraception 2003;67(1):1‐8. - PubMed
Goldberg 2007
-
- Goldberg AB, Grimes DA. Injectable contraceptives. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Stewart F, Kowal D editor(s). Contraceptive Technology. 19th Edition. New York: Ardent Media, Inc., 2007:157‐179.
IPPF 2010
-
- IPPF Directory of Hormonal Contraceptives. Available at http://contraceptive.ippf.org. (accessed 23 Sep 2010).
Kaunitz 2000
-
- Kaunitz AM. Injectable contraception. New and existing options. Obstetrics and Gynecology Clinics of North America 2000;27(4):741‐80. - PubMed
Kaunitz 2001
-
- Kaunitz AM. Lunelle monthly injectable contraceptive. An effective, safe, and convenient new birth control option. Archives of Gynecology and Obstetrics 2001;265(3):119‐23. - PubMed
Koetsawang 1994
-
- Koetsawang S. Once‐a‐month injectable contraceptives: efficacy and reasons for discontinuation. Contraception 1994;49(4):387‐98. - PubMed
Maderas 2007
-
- Monastersky Maderas NJ, Landau SC. Pharmacy and clinic partnerships to expand access to injectable contraception. Journal of the American Pharmacists Association (2003) 2007;47(4):527‐31. - PubMed
Newton 1994
-
- Newton JR, d'Arcangues C, Hall PE. A review of "once‐a‐month" combined injectable contraceptives. Journal of Obstetrics and Gynaecology 1994;4(Suppl 1):1‐34. - PubMed
Pardthaisong 1980
-
- Pardthaisong T, Gray RH, McDaniel EB. Return of fertility after discontinuation of depot medroxyprogesterone acetate and intra‐uterine devices in Northern Thailand. Lancet 1980;1(8167):509‐12. - PubMed
PharmacyAccess 2005
-
- Pharmacy Access Partnership. Injectable Contraceptive (IC) Program. www.pharmacyaccess.org/ICProgram.htm (accessed 30 March 2005).
Schwallie 1974
-
- Schwallie PC, Assenzo JR. The effect of depo‐medroxyprogesterone acetate on pituitary and ovarian function, and the return of fertility following its discontinuation: a review. Contraception 1974;10(2):181‐202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

