Drugs for treating uncomplicated malaria in pregnant women
- PMID: 18843672
- PMCID: PMC6532683
- DOI: 10.1002/14651858.CD004912.pub3
Drugs for treating uncomplicated malaria in pregnant women
Abstract
Background: Women are more vulnerable to malaria during pregnancy, and malaria infection may have adverse consequences for the fetus. Identifying safe and effective treatments is important.
Objectives: To compare the effects of drug regimens for treating uncomplicated falciparum malaria in pregnant women.
Search strategy: We searched the Cochrane Infectious Diseases Group Specialized Register (February 2008), CENTRAL (The Cochrane Library 2008, Issue 1), MEDLINE (1966 to February 2008), EMBASE (1974 to February 2008), LILACS (February 2008), mRCT (February 2008), reference lists, and conference abstracts. We also contacted researchers in the field, organizations, and pharmaceutical companies.
Selection criteria: Randomized and quasi-randomized controlled trials of antimalarial drugs for treating uncomplicated malaria in pregnant women.
Data collection and analysis: Two authors assessed trial eligibility and risk of bias, and extracted data. We performed a quantitative analysis only where we could combine the data. We combined dichotomous data using the risk ratio (RR) and presented each result with a 95% confidence interval (CI).
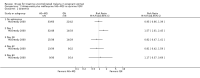
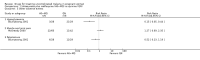
Main results: Ten trials (1805 participants) met the inclusion criteria. Two were quasi-randomized, seven did not describe allocation concealment, and all adjusted treatment failure to exclude new infections. One trial reported fewer treatment failures at day 63 with artesunate plus mefloquine compared with quinine (RR 0.09, 95% CI 0.02 to 0.38; 106 participants). One trial reported fewer treatment failures at day 63 with artesunate plus atovaquone-proguanil compared with quinine (RR 0.14, 95% CI 0.03 to 0.57; 80 participants). One trial reported fewer treatment failures at day 28 when amodiaquine was compared with chloroquine (RR 0.20, 95% CI 0.08 to 0.46; 420 participants) and when amodiaquine plus sulfadoxine-pyrimethamine was compared with chloroquine (RR 0.02, 95% CI 0.00 to 0.26; 418 participants). Compared with sulfadoxine-pyrimethamine given alone, one trial reported fewer treatment failures at delivery (or day 40) with artesunate plus sulfadoxine-pyrimethamine (RR 0.15, 95% CI 0.04 to 0.59; 79 participants) and azithromycin plus sulfadoxine-pyrimethamine (RR 0.27, 95% CI 0.10 to 0.76; 82 participants).
Authors' conclusions: Data are scant. Some combination treatments appear to be effective at treating malaria in pregnancy; however, safety data are limited.
Conflict of interest statement
None known.
Figures
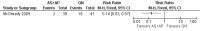
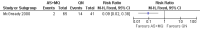
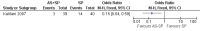
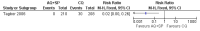
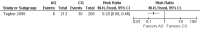
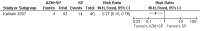





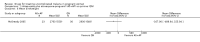

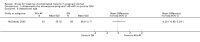






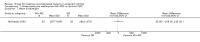














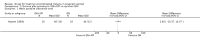
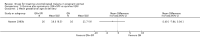




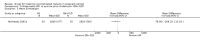
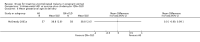
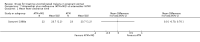
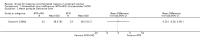

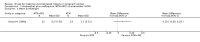

























Update of
-
Drugs for treating uncomplicated malaria in pregnant women.Cochrane Database Syst Rev. 2005 Jul 20;(3):CD004912. doi: 10.1002/14651858.CD004912.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2008 Oct 08;(4):CD004912. doi: 10.1002/14651858.CD004912.pub3. PMID: 16034957 Updated.
References
References to studies included in this review
Bounyasong 2001 {published data only}
-
- Bounyasong S. Randomized trial of artesunate and mefloquine in comparison with quinine sulphate to treat P. falciparum malaria pregnant women. Journal of the Medical Association of Thailand 2001;84(9):1289‐98. - PubMed
Coulibaly 2006 {published data only}
Kalilani 2007 {published data only}
Mbanzulu 1993 {published data only}
-
- Mbanzulu PN, Tona L, Nekwei W, Kobota V, Kisile M, Makengo M. Management of malaria during pregnancy in Kinshasa (Zaire) by chloroquine and clindamycin [Traitment du paludisme de la femme enceinte a Kinshasa (Republique democratique du Congo) par la chloroquine et la clinadmycine]. Revue Francaise de Gynecologie et d'Obstetrique 1998;93(6):433‐7.
McGready 2000 {published data only}
-
- McGready R, Brockman A, Cho T, Cho D, Vugt M, Luxemburger C, et al. Randomized comparison of mefloquine‐artesunate versus quinine in the treatment of multidrug‐resistant falciparum malaria in pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(6):689‐93. - PubMed
McGready 2001a {published data only}
-
- McGready R, Cho T, Samuel, Villegas L, Brockman A, Vugt M, et al. Randomized comparison of quinine‐clindamycin versus artesunate in the treatment of falciparum malaria in pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(6):651‐6. - PubMed
McGready 2005 {published data only}
-
- McGready R, Ashley EA, Moo E, Cho T, Barends M, Hutagalung R, et al. A randomized comparison of artesunate‐atovaquone‐proguanil versus quinine in treatment for uncomplicated falciparum malaria during pregnancy. Journal of Infectious Diseases 2005;192(5):846‐53. - PubMed
Nosten 1993b {published data only}
-
- Nosten F, ter Kuile F, Thwai KL, Maelankirri L, White NJ. Spriamycin does not potentiate quinine treatment of falciparum malaria in pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87(3):305‐6. - PubMed
Sowunmi 1998a {published data only}
-
- Sowunmi A, Oduola AMJ, Ogundahunsi OAT, Fehintola FA, Ilesanmi OA, Akinyinka OO, et al. Randomised trial of artemether versus artemether and mefloquine for the treatment of chloroquine/sulphadoxine‐pyrimethamine‐resistant falciparum malaria during pregnancy. Journal of Obstetrics and Gynaecology 1998;18(4):322‐7. - PubMed
Tagbor 2006 {published data only}
-
- Tagbor H, Bruce J, Browne E, Randal A, Greenwood B, Chandramohan D. Efficacy, safety, and tolerability of amodiaquine plus sulphadoxine‐pyrimethamine used alone or in combination for malaria treatment in pregnancy: a randomised trial. Lancet 2006;368(9544):1349‐56. - PubMed
-
- Tagbor H, Bruce J, Ord R, Randall A, Browne E, Greenwood B, et al. Comparison of the therapeutic efficacy of chloroquine and sulphadoxine‐pyremethamine in children and pregnant women. Tropical Medicine and International Health 2007;12(11):1288‐97. - PubMed
References to studies excluded from this review
Deen 2001 {published data only}
-
- Deen JL, Seidlein L, Pinder M, Walraven GEL, Greenwood BM. The safety of the combination artesunate and pyrimethamine‐sulphadoxine given during pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(4):424‐8. - PubMed
Keuter 1990 {published data only}
McGready 2001b {published data only}
-
- McGready R, Cho T, Keo NK, Thwai KL, Villegas L, Looaresuwan S, et al. Artemisinin antimalarials in pregnancy: a prospective treatment study of 539 episodes of multidrug‐resistant Plasmodium falciparum. Clinical Infectious Diseases 2001;33(12):2009‐16. - PubMed
Naing 1998 {published data only}
-
- Naing T, Win H, Nwe YY. Falciparum malaria and pregnancy: relationship and treatment response. Southeast Asian Journal of Tropical Medicine and Public Health 1998;19(2):253‐8. - PubMed
Ndyomugyenyi 2000 {published data only}
-
- Ndyomugyenyi R, Magnussen P. Chloroquine prophylaxis, iron/folic‐acid supplementation or case management of malaria attacks in primigravidae in western Uganda: effects on congenital malaria and infant haemoglobin concentrations. Annals of Tropical Medicine and Parasitology 2000;94(8):759‐70. - PubMed
Sowunmi 1998b {published data only}
-
- Sowunmi A, Ilesanmi AO, Oduloa AMJ, Omitowoju GO, Ojengbede OA. Efficacy of mefloquine in uncomplicated chloroquine‐resistant falciparum malaria during pregnancy. Journal of Obstetrics and Gynaecology 1998;16(5):362‐3.
Steketee 1996 {published data only}
-
- Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, Breman JG. The effect of malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. American Journal of Tropical Medicine and Hygiene 1996;55 Suppl 1:33‐41. - PubMed
-
- Steketee RW, Wirima JJ, Slutsker L, Breman JG, Heymann DL. Comparability of treatment groups and risk factors for parasitemia at the first antenatal clinic visit in a study of malaria treatment and prevention in pregnancy in rural Malawi. American Journal of Tropical Medicine and Hygiene 1996;55 Suppl 1:17‐23. - PubMed
-
- Steketee RW, Wirima JJ, Slutsker L, Khoromana CO, Heymann DL, Breman JG. Malaria treatment and prevention in pregnancy: indications for use and adverse events associated with use of chloroquine and mefloquine. American Journal of Tropical Medicine and Hygiene 1996;55 Suppl 1:50‐6. - PubMed
-
- Steketee RW, Wirima JJ, Slutsker L, Roberts JM, Khoromana O, Heymann DL. Malaria parasite infection during pregnancy and at delivery in mother, placenta, and newborn: efficacy of chloroquine and mefloquine in rural Malawi. American Journal of Tropical Medicine and Hygiene 1996;55 Suppl 1:24‐32. - PubMed
-
- Steketee RW, Wirima JJ, Slutsker WL, Khoromana CO, Breman JG, Heymann DL. Objectives and methodology in a study of malaria treatment and prevention in pregnancy in rural Malawi: The Mangochi Malaria Research Project. American Journal of Tropical Medicine and Hygiene 1996;55 Suppl 1:8‐16. - PubMed
References to ongoing studies
ISRCTN86353884 {published data only}
-
- ISRCTN86353884. Co‐Artemether in pregnancy ‐ a pilot study (Thailand). www.controlled‐trials.com/ISRCTN86353884 (accessed February 2008).
Additional references
Adjuik 2004
-
- Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N, International Artemisinin Study Group. Artesunate combinations for treatment of malaria: meta‐analysis. Lancet 2004;363(9402):9‐17. - PubMed
Brabin 2001
-
- Brabin BJ, Rogerson SJ. The epidemiology and outcomes of maternal malaria. In: Duffy PE, Fried M editor(s). Malaria in pregnancy: deadly parasite, susceptible host. London and New York: Taylor & Francis, 2001:27‐52.
Clyde 1981
Duffy 2001
-
- Duffy PE. Immunity to malaria during pregnancy: different host, different parasite. In: Duffy PE, Fried M editor(s). Malaria in pregnancy: deadly parasite, susceptible host. London: Taylor & Francis, 2001:71‐127.
Gamble 2006
Garner 2006
Guerin 2002
-
- Guerin PJ, Olliaro P, Nosten F, Druilhe P, Laxminarayan R, Binka F, et al. Malaria: current status of control, diagnosis, treatment, and a proposed agenda for research and development. Lancet Infectious Diseases 2002;2(9):564‐73. - PubMed
Hoffman 1992
-
- Hoffman SL. Diagnosis, treatment, and prevention of malaria. Medical Clinics of North America 1992;76(6):1327‐55. - PubMed
Jüni 2001
Lefebvre 2008
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Lindsay 2000
-
- Lindsay S, Ansell J, Selman C, Cox V, Hamilton K, Walraven G. Effect of pregnancy on exposure to malaria mosquitoes. Lancet 2000;355(9219):1972. - PubMed
Menendez 2000
-
- Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, et al. The impact of placental malaria on gestational age and birth weight. Journal of Infectious Diseases 2000;181(5):1740‐5. - PubMed
Nosten 1993a
-
- Nosten F, ter Kuile FO, Luxemberger C, Woodrow C, Kyle DE, Chongsuphajaisiddhi T, et al. Cardiac effects of antimalarial treatment with halofantrine. Lancet 1993;341(8852):1054‐6. - PubMed
Nosten 1999
-
- Nosten F, Vincenti M, Simpson J, Yei P, Thwai KL, Vries A, et al. The effects of mefloquine treatment in pregnancy. Clinical Infectious Diseases 1999;28(4):808‐15. - PubMed
Nosten 2001
-
- Nosten F, McGready R. The treatment of malaria in pregnancy. In: Duffy PE, Fried M editor(s). Malaria in pregnancy: deadly parasite, susceptible host. London: Taylor & Francis, 2001:223‐41.
RBM 2003
-
- Global Partnership to Roll Back Malaria, UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Assessment of the safety of artemisinin compounds in pregnancy: report of two informal consultations convened by WHO in 2002. Geneva: World Health Organization, 2003.
RBM 2005a
-
- Global Partnership to Roll Back Malaria. World malaria report: 2005. Geneva: World Health Organization, 2005.
RBM 2005b
-
- Roll Back Malaria (RBM) Partnership. Global Strategic Plan 2005‐2015. www.rollbackmalaria.org/forumV/docs/gsp_en.pdf November 2005 (accessed 1 May 2008).
Review Manager 5 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Steketee 2001
-
- Steketee RE, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria‐endemic areas. American Journal of Tropical Medicine and Hygiene 2001;64 Suppl 1‐2:28‐35. - PubMed
Stevens 2000
-
- Stevens RD. Anaemia ‐‐ the scourge of the Third World. Health Millions 2000;26(2):21‐3. - PubMed
Taylor 2004
-
- Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Safety 2004;27(1):25‐61. - PubMed
White 1999
-
- White NJ, Nosten F, Looareesuwan S, Watkins WM, Mrash K, Snow RW, et al. Averting a malaria disaster. Lancet 1999;353(9168):1965‐7. - PubMed
WHO 2003a
-
- World Health Organization, Global Partnership to Roll Back Malaria. The Abuja Declaration and the plan of action: an extract from the African Summit on Roll Back Marlaria, Abuja, 25 April 2000 [WHO/CDS/RBM/2000.17]. Geneva: World Health Organization, 2003.
WHO 2003b
-
- World Health Organization. Lives at risk: malaria in pregnancy. www.who.int/features/2003/04b/en (accessed December 2004).
WHO 2003c
-
- Global Partnership to Roll Back Malaria. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparium malaria. Geneva: World Health Organization, 2003.
WHO 2004a
-
- World Health Organization, Regional Office for Africa. A strategic framework for malaria prevention and control during pregnancy in the African region. Brazzaville: World Health Organization, 2004.
WHO 2004b
-
- World Health Organization, Regional Office for Africa. Malaria control in Africa: progress report on implementation of the plan of action of the Abuja Declaration. Brazzaville: WHO Regional Office for Africa, 2004.
WHO 2006
-
- World Health Organization Roll Back Malaria Department. Guidelines for the treatment of malaria [WHO/HTM/MAL/2006.1108]. Geneva: World Health Organization, 2006.
WHO 2007
-
- World Health Organization Global Malaria Programme. Anti‐malarial drug policies: AFRO, AMRO, EMRO, EURO, SEARO, WPRO. www.who.int/malaria/treatmentpolicies.html Updated April 2007 (accessed August 2007).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

