Mitochondrial iron accumulation with age and functional consequences
- PMID: 18843794
- PMCID: PMC3849824
- DOI: 10.1111/j.1474-9726.2008.00418.x
Mitochondrial iron accumulation with age and functional consequences
Abstract
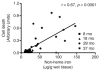
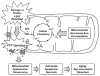
During the aging process, an accumulation of non-heme iron disrupts cellular homeostasis and contributes to the mitochondrial dysfunction typical of various neuromuscular degenerative diseases. Few studies have investigated the effects of iron accumulation on mitochondrial integrity and function in skeletal muscle and liver tissue. Thus, we isolated liver mitochondria (LM), as well as quadriceps-derived subsarcolemmal mitochondria (SSM) and interfibrillar mitochondria (IFM), from male Fischer 344 x Brown Norway rats at 8, 18, 29 and 37 months of age. Non-heme iron content in SSM, IFM and LM was significantly higher with age, reaching a maximum at 37 months of age. The mitochondrial permeability transition pore (mPTP) was more susceptible to the opening in aged mitochondria containing high levels of iron (i.e. SSM and LM) compared to IFM. Furthermore, mitochondrial RNA oxidation increased significantly with age in SSM and LM, but not in IFM. Levels of mitochondrial RNA oxidation in SSM and LM correlated positively with levels of mitochondrial iron, whereas a significant negative correlation was observed between the maximum Ca(2+) amounts needed to induce mPTP opening and iron contents in SSM, IFM and LM. Overall, our data suggest that age-dependent accumulation of mitochondrial iron may increase mitochondrial dysfunction and oxidative damage,thereby enhancing the susceptibility to apoptosis.
Figures

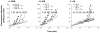
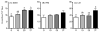



References
-
- Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA. Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am J Physiol Cell Physiol. 2005;289:C994–C1001. - PubMed
-
- Atamna H, Walter PB, Ames BN. The role of heme and iron-sulfur clusters in mitochondrial biogenesis, maintenance, and decay with age. Arch Biochem Biophys. 2002;397:345–353. - PubMed
-
- Babcock M, de SD, Oaks R, vis-Kaplan S, Jiralerspong S, Montermini L, Pandolfo M, Kaplan J. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science. 1997;276:1709–1712. - PubMed
-
- Beinert H, Kiley PJ. Fe-S proteins in sensing and regulatory functions. Curr Opin Chem Biol. 1999;3:152–157. - PubMed
-
- Brunner EA, Cheng SC, Berman ML. Effects of anesthesia on intermediary metabolism. Annu Rev Med. 1975;26:391–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

