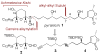
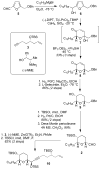
A concise and modular synthesis of pyranicin
- PMID: 18844364
- PMCID: PMC2901595
- DOI: 10.1021/ol802041c
A concise and modular synthesis of pyranicin
Abstract
A modular, 13-step synthesis of the tetrahydropyran-containing annonaceous acetogenin pyranicin is reported. Key features are the use of an Achmatowicz oxidation-Kishi reduction sequence for the assembly of a pyranone from a furan and the application of Fu's alkyl-alkyl Suzuki coupling for subunit union.
Figures
References
-
-
For a review see: Bermejo A, Figadere B, Zafra-Polo M-C, Barrachina I, Estornell E, Cortes D. Nat Prod Rep. 2005;22:269. and references cited therein.
-
-
- Alali FQ, Rogers L, Zhang Y, McLaughlin JL. Tetrahedron. 1998;54:5833.
-
- Keaton KA, Phillips AJ. J Am Chem Soc. 2006;128:408. - PubMed
-
-
For other total syntheses see: Strand D, Norrby PO, Rein T. J Org Chem. 2006;71:1879.Strand D, Rein T. Org Lett. 2005;7:199.Takahashi S, Kubota A, Nakata T. Org Lett. 2003;5:1353.Hattori Y, Furuhata S-i, Okajima M, Konno H, Abe M, Miyoshi H, Goto T, Makabe H. Org Lett. 2008;10:717.Furuhata S-i, Hattori Y, Okajima M, Konno H, Abe M, Miyoshi H, Goto T, Makabe H. Tetrahedron. 2008;64:7695.
-
-
-
Henderson JA, Jackson KL, Phillips AJ. Org Lett. 2007;9:5299.Um JM, Houk KN, Phillips AJ. Org Lett. 2008;10:3769.See also Nicolaou KC, Frederick MO, Burtoloso ACB, Denton RM, Rivas F, Cole KP, Aversa RJ, Gibe R, Umezawa T, Suzuki TJ. Am Chem Soc. 2008;130:7466.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources