Pneumococcal vaccination of elderly adults: new paradigms for protection
- PMID: 18844484
- PMCID: PMC6364558
- DOI: 10.1086/592691
Pneumococcal vaccination of elderly adults: new paradigms for protection
Abstract
Pneumococcal polysaccharide vaccine has been licensed for use in the United States for >30 years, and two-thirds of the elderly population in the United States have received this vaccine. Observational studies have demonstrated that pneumococcal polysaccharide vaccine reduces the risk of invasive pneumococcal disease in immunocompetent elderly individuals, but neither observational studies nor clinical trials have demonstrated consistent evidence for a reduction in the incidence of pneumonia in vaccinated older adults. The introduction of pneumococcal protein conjugate vaccine among children has led to a herd immunity effect that has resulted in a 38% decrease in the rate of invasive pneumococcal disease among elderly adults. The high efficacy of pneumococcal protein conjugate vaccine in children has renewed interest in evaluating pneumococcal protein conjugate vaccines in adults for prevention of invasive pneumococcal disease and pneumonia. Moreover, the recognition of the presence and function of noncapsular pneumococcal protein antigens and the increasing availability of adjuvants highlight the promise of new vaccination strategies to decrease the burden of pneumococcal infection in this high-risk population.
Conflict of interest statement
Figures

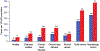

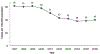

References
-
- Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Questionnaire US Department of Health and Human Services. Available at: http://www.cdc.gov/brfss/questionnaires/english.htm. Accessed 25 September 2008.
-
- Shapiro ED, Clemens JD. A controlled evaluation of the protective efficacy of pneumococcal vaccine for patients at high risk of serious pneumococcal infections. Ann Intern Med 1984; 101:325–30. - PubMed
-
- Forrester HL, Jahnigen DW, LaForce FM. Inefficacy of pneumococcal vaccine in a high-risk population. Am J Med 1987; 83:425–30. - PubMed
-
- Sims RV, Steinmann WC, McConville JH, King LR, Zwick WC, Schwartz JS. The clinical effectiveness of pneumococcal vaccine in the elderly. Ann Intern Med 1988; 108:653–7. - PubMed
-
- Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med 1991; 325:1453–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

