Stability study of unmodified siRNA and relevance to clinical use
- PMID: 18844576
- PMCID: PMC2829675
- DOI: 10.1089/oli.2008.0149
Stability study of unmodified siRNA and relevance to clinical use
Abstract
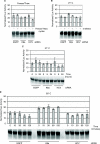
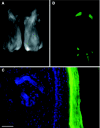
RNA interference offers enormous potential to develop therapeutic agents for a variety of diseases. To assess the stability of siRNAs under conditions relevant to clinical use with particular emphasis on topical delivery considerations, a study of three different unmodified siRNAs was performed. The results indicate that neither repeated freeze/thaw cycles, extended incubations (over 1 year at 21 degrees C), nor shorter incubations at high temperatures (up to 95 degrees C) have any effect on siRNA integrity as measured by nondenaturing polyacrylamide gel electrophoresis and functional activity assays. Degradation was also not observed following exposure to hair or skin at 37 degrees C. However, incubation in fetal bovine or human sera at 37 degrees C led to degradation and loss of activity. Therefore, siRNA in the bloodstream is likely inactivated, thereby limiting systemic exposure. Interestingly, partial degradation (observed by gel electrophoresis) did not always correlate with loss of activity, suggesting that partially degraded siRNAs retain full functional activity. To demonstrate the functional activity of unmodified siRNA, EGFP-specific inhibitors were injected into footpads and shown to inhibit preexisting EGFP expression in a transgenic reporter mouse model. Taken together, these data indicate that unmodified siRNAs are viable therapeutic candidates.
Figures



References
-
- BITKO V. MUSIYENKO A. SHULYAYEVA O. BARIK S. Inhibition of respiratory viruses by nasally administered siRNA. Nat. Med. 2005;11:50–55. - PubMed
-
- BRAASCH D.A. JENSEN S. LIU Y. KAUR K. ARAR K. WHITE M.A. COREY D.R. RNA interference in mammalian cells by chemically-modified RNA. Biochemistry. 2003;42:7967–7975. - PubMed
-
- CAO Y.A. BACHMANN M.H. BEILHACK A. YANG Y. TANAKA M. SWIJNENBURG R.J. REEVES R. TAYLOR-EDWARDS C. SCHULZ S. DOYLE T.C. FATHMAN C.G. ROBBINS R.C. HERZENBERG L.A. NEGRIN R.S. CONTAG C.H. Molecular imaging using labeled donor tissues reveals patterns of engraftment, rejection, and survival in transplantation. Transplantation. 2005;80:134–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

