Optical spectroscopy and imaging for the noninvasive evaluation of engineered tissues
- PMID: 18844604
- PMCID: PMC2817652
- DOI: 10.1089/ten.teb.2008.0248
Optical spectroscopy and imaging for the noninvasive evaluation of engineered tissues
Abstract
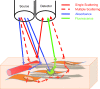
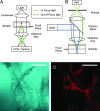
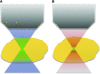
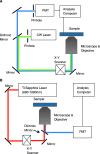
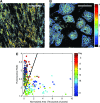
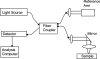
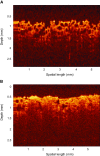
Optical spectroscopy and imaging approaches offer the potential to noninvasively assess different aspects of the cellular, extracellular matrix, and scaffold components of engineered tissues. In addition, the combination of multiple imaging modalities within a single instrument is highly feasible, allowing acquisition of complementary information related to the structure, organization, biochemistry, and physiology of the sample. The ability to characterize and monitor the dynamic interactions that take place as engineered tissues develop promises to enhance our understanding of the interdependence of processes that ultimately leads to functional tissue outcomes. It is expected that this information will impact significantly upon our abilities to optimize the design of biomaterial scaffolds, bioreactors, and cell systems. Here, we review the principles and performance characteristics of the main methodologies that have been exploited thus far, and we present examples of corresponding tissue engineering studies.
Figures




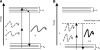
References
-
- Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920–926. - PubMed
-
- Pancrazio J.J. Wang F. Kelley C.A. Enabling tools for tissue engineering. Biosens Bioelectron. 2007;22:2803–2811. - PubMed
-
- Fang H., et al. Noninvasive sizing of subcellular organelles with light scattering spectroscopy. IEEE J Sel Top Quantum Electron. 2003;9:267–276.
-
- Backman V., et al. Measuring cellular structure at submicrometer scale with light scattering spectroscopy. IEEE J Sel Top Quantum Electron. 2001;7:887–893.
-
- Kim Y., et al. Simultaneous measurement of angular and spectral properties of light scattering for characterization of tissue microarchitecture and its alteration in early precancer. IEEE J Sel Top Quantum Electron. 2003;9:243–256.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

