Can oligomeric T-cell receptor be used as a tool to detect viral peptide epitopes on infected cells?
- PMID: 18845488
- PMCID: PMC2632680
- DOI: 10.1016/j.clim.2008.08.025
Can oligomeric T-cell receptor be used as a tool to detect viral peptide epitopes on infected cells?
Abstract
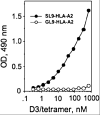
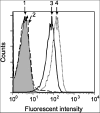
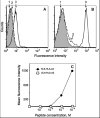
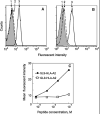
We have utilized soluble HIV Gag-specific T-cell receptor (TCR) D3 with low affinity and TCR-like antibody 25-D1.16 recognizing its natural peptide-MHC (pMHC) ligand with high affinity to determine how affinity and off-rate of the receptor-pMHC interactions affect the sensitivity of pMHC detection on the cell surface. We found that with soluble TCR cognate pMHCs can be detected only at relatively high cell surface densities when the TCR was oligomerized using either Streptavidin or quantum dot (QD) scaffolds. While the higher affinity probe led to a greater sensitivity of pMHC detection, monomers and oligomers of the probe showed essentially the same detection limit, which is restricted by the sensitivity of standard flow cytometry technique. We have also shown that imaging of QD/TCR specifically bound to cognate pMHC on the cell surface yielded a very bright fluorescent signal that can enhance the sensitivity of viral peptide detection on infected cells.
Figures


References
-
- Eisen HN, Sykulev Y, Tsomides TJ. The antigen-specific T-cell receptor and its reactions with peptide-MHC complexes. In: Haber E, editor. Antigen-Binding Molecules Antibodies and T-cell Receptors. Academic Press; San Diego: 1997. pp. 1–56.
-
- Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annual Review of Immunology. 1998;16:523–44. - PubMed
-
- Sykulev Y, Anikeeva N, Kalams SA. Soluble TCR/tetramer as a tool to study the distribution of immunodominant HIV Gag peptide epitope on the surface of target cells. Experimental Biology Meeting; New Orlean, LA. 2002.
-
- Subbramanian RA, Moriya C, Martin KL, Peyerl FW, Hasegawa A, Naoi A, Chhay H, Autissier P, Gorgone DA, Lifton MA, Kuus-Reichel K, Schmitz JE, Letvin NL, Kuroda MJ. Engineered T-cell receptor tetramers bind MHC-peptide complexes with high affinity. Nat Biotechnol. 2004;22:1429–34. - PubMed
-
- Laugel B, Boulter JM, Lissin N, Vuidepot A, Li Y, Gostick E, Crotty LE, Douek DC, Hemelaar J, Price DA, Jakobsen BK, Sewell AK. Design of soluble recombinant T cell receptors for antigen targeting and T cell inhibition. J Biol Chem. 2005;280:1882–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

