Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia
- PMID: 18845557
- PMCID: PMC2879685
- DOI: 10.1093/schbul/sbn131
Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia
Abstract
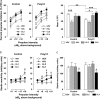
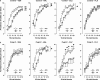
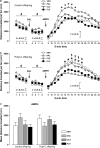
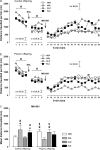
Current pharmacotherapy of schizophrenia remains unsatisfactory with little hope for complete functional restoration in patients once the disease has developed. A preventive approach based on intervention in the prodromal stage of the disease aiming to preserve functional integrity by halting the progress of the disease is therefore extremely attractive. Here, we investigated the effects of preventive antipsychotic or antidepressant drug treatment in a well-established neurodevelopmental mouse model of multiple schizophrenia-related abnormalities. Pregnant mice on gestation day 9 were exposed to the viral mimic polyriboinosinic-polyribocytidylic acid (2 mg/kg, intravenously) or corresponding vehicle treatment, and the resulting offspring from both prenatal treatment conditions were subjected to chronic antipsychotic (haloperidol or clozapine), antidepressant (fluoxetine), or placebo treatment during the periadolescent stage of development. The effects of the preventive pharmacotherapy on behavioral and pharmacological functions were then investigated in adulthood using paradigms relevant to schizophrenia, namely prepulse inhibition, latent inhibition, and sensitivity to psychostimulant drugs. We show that periadolescent treatment with the reference antipsychotic and antidepressant drugs can successfully block the emergence of multiple psychosis-related behavioral and pharmacological abnormalities in subjects predisposed to adult brain pathology by exposure to prenatal immune challenge. At the same time, however, our study reveals numerous negative influences of the early pharmacological intervention on normal behavioral development in control subjects. Hence, even though preventive pharmacotherapy may be beneficial in individuals with predisposition to psychosis-related brain dysfunctions, chronic antipsychotic or antidepressant drug treatment in false-positive subjects is associated with substantial risk for long-term behavioral disturbances in adulthood.
Figures
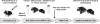
Similar articles
-
Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia.Neurosci Biobehav Rev. 2005;29(6):913-47. doi: 10.1016/j.neubiorev.2004.10.012. Neurosci Biobehav Rev. 2005. PMID: 15964075 Review.
-
Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia.Biol Psychiatry. 2009 Dec 1;66(11):1038-46. doi: 10.1016/j.biopsych.2009.07.005. Epub 2009 Sep 2. Biol Psychiatry. 2009. PMID: 19726031
-
Tracing the development of psychosis and its prevention: what can be learned from animal models.Neuropharmacology. 2012 Mar;62(3):1273-89. doi: 10.1016/j.neuropharm.2011.04.019. Epub 2011 Jun 23. Neuropharmacology. 2012. PMID: 21703648 Review.
-
Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia.Biol Psychiatry. 2006 Mar 15;59(6):546-54. doi: 10.1016/j.biopsych.2005.07.031. Epub 2005 Oct 26. Biol Psychiatry. 2006. PMID: 16256957
-
Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice.Brain Behav Immun. 2008 May;22(4):469-86. doi: 10.1016/j.bbi.2007.09.012. Epub 2007 Nov 26. Brain Behav Immun. 2008. PMID: 18023140
Cited by
-
Antipsychotic-induced sensitization and tolerance: Behavioral characteristics, developmental impacts, and neurobiological mechanisms.J Psychopharmacol. 2016 Aug;30(8):749-70. doi: 10.1177/0269881116654697. Epub 2016 Jul 1. J Psychopharmacol. 2016. PMID: 27371498 Free PMC article. Review.
-
Risperidone administered during asymptomatic period of adolescence prevents the emergence of brain structural pathology and behavioral abnormalities in an animal model of schizophrenia.Schizophr Bull. 2011 Nov;37(6):1257-69. doi: 10.1093/schbul/sbq040. Epub 2010 May 3. Schizophr Bull. 2011. PMID: 20439320 Free PMC article.
-
Epigallocatechin Gallate (EGCG), a Green Tea Polyphenol, Reduces Coronavirus Replication in a Mouse Model.Viruses. 2021 Dec 17;13(12):2533. doi: 10.3390/v13122533. Viruses. 2021. PMID: 34960802 Free PMC article.
-
Prenatal immune challenge in rats: altered responses to dopaminergic and glutamatergic agents, prepulse inhibition of acoustic startle, and reduced route-based learning as a function of maternal body weight gain after prenatal exposure to poly IC.Synapse. 2012 Aug;66(8):725-37. doi: 10.1002/syn.21561. Epub 2012 May 15. Synapse. 2012. PMID: 22473973 Free PMC article.
-
Behavioral alterations in rat offspring following maternal immune activation and ELR-CXC chemokine receptor antagonism during pregnancy: implications for neurodevelopmental psychiatric disorders.Prog Neuropsychopharmacol Biol Psychiatry. 2015 Mar 3;57:155-65. doi: 10.1016/j.pnpbp.2014.11.002. Epub 2014 Nov 12. Prog Neuropsychopharmacol Biol Psychiatry. 2015. PMID: 25445065 Free PMC article.
References
-
- Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164. - PubMed
-
- McGlashan TH. Early detection and intervention in schizophrenia: research. Schizophr Bull. 1996;22:327–345. - PubMed
-
- Cornblatt B, Lencz T, Obuchowski M. The schizophrenia prodrome: treatment and high-risk perspectives. Schizophr Res. 2002;54:177–186. - PubMed
-
- Ruhrmann S, Schultze-Lutter F, Maier W, Klosterkötter J. Pharmacological intervention in the initial prodromal phase of psychosis. Eur Psychiatry. 2005;20:1–6. - PubMed
-
- Woods SW, Breier A, Zipursky RB, et al. Randomized trial of olanzapine versus placebo in the symptomatic acute treatment of the schizophrenic prodrome. Biol Psychiatry. 2003;54:453–464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

