Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue
- PMID: 18845643
- PMCID: PMC2646525
- DOI: 10.1210/en.2008-1118
Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue
Abstract
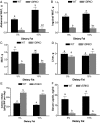
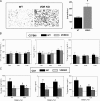
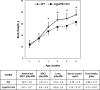
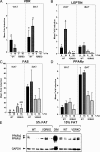
Increased adiposity is a feature of aging in both mice and humans, but the molecular mechanisms underlying age-related changes in adipose tissue stores remain unclear. In previous studies, we noted that 18-month-old normocalcemic vitamin D receptor (VDR) knockout (VDRKO) mice exhibited atrophy of the mammary adipose compartment relative to wild-type (WT) littermates, suggesting a role for VDR in adiposity. Here we monitored body fat depots, food intake, metabolic factors, and gene expression in WT and VDRKO mice on the C57BL6 and CD1 genetic backgrounds. Regardless of genetic background, both sc and visceral white adipose tissue depots were smaller in VDRKO mice than WT mice. The lean phenotype of VDRKO mice was associated with reduced serum leptin and compensatory increased food intake. Similar effects on adipose tissue, leptin and food intake were observed in mice lacking Cyp27b1, the 1alpha-hydroxylase enzyme that generates 1,25-dihydroxyvitamin D(3), the VDR ligand. Although VDR ablation did not reduce expression of peroxisome proliferator-activated receptor-gamma or fatty acid synthase, PCR array screening identified several differentially expressed genes in white adipose tissue from WT and VDRKO mice. Uncoupling protein-1, which mediates dissociation of cellular respiration from energy production, was greater than 25-fold elevated in VDRKO white adipose tissue. Consistent with elevation in uncoupling protein-1, VDRKO mice were resistant to high-fat diet-induced weight gain. Collectively, these studies identify a novel role for 1,25-dihydroxyvitamin D(3) and the VDR in the control of adipocyte metabolism and lipid storage in vivo.
Figures


References
-
- Kalaany NY, Gauthier KC, Zavacki AM, Mammen PP, Kitazume T, Peterson JA, Horton JD, Garry DJ, Bianco AC, Mangelsdorf DJ 2005 LXRs regulate the balance between fat storage and oxidation. Cell Metab 1:231–244 - PubMed
-
- Kamei Y, Kawada T, Kazuki R, Ono T, Kato S, Sugimoto E 1993 Vitamin D receptor gene expression is up-regulated by 1, 25-dihydroxyvitamin D3 in 3T3-L1 preadipocytes. Biochem Biophys Res Commun 193:948–955 - PubMed
-
- Imagawa M, Tsuchiya T, Nishihara T 1999 Identification of inducible genes at the early stage of adipocyte differentiation of 3T3-L1 cells. Biochem Biophys Res Commun 254:299–305 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

