Methionine in proteins defends against oxidative stress
- PMID: 18845767
- PMCID: PMC2630790
- DOI: 10.1096/fj.08-118414
Methionine in proteins defends against oxidative stress
Abstract
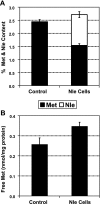
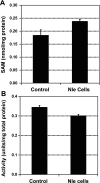
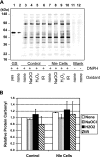
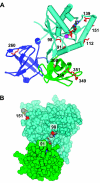
A variety of reactive oxygen species react readily with methionine residues in proteins to form methionine sulfoxide, thus scavenging the reactive species. Most cells contain methionine sulfoxide reductases, which catalyze a thioredoxin-dependent reduction of methionine sulfoxide back to methionine. Thus, methionine residues may act as catalytic antioxidants, protecting both the protein where they are located and other macromolecules. To test this hypothesis directly, we replaced 40% of the methionine residues in Escherichia coli with norleucine, the carbon-containing analog, in which the sulfur of methionine is substituted by a methylene group (-CH2-). The intracellular free methionine and S-adenosylmethionine pools were not altered, nor was the specific activity of the key enzyme, glutamine synthetase. When unstressed, both control and norleucine-substituted cells survived equally well at stationary phase for at least 32 h. However, oxidative stress was more damaging to the norleucine-substituted cells. They died more rapidly than control cells when challenged by hypochlorite, hydrogen peroxide, or ionizing radiation. One of the most abundant proteins in the cell, elongation factor Tu, was found to be more oxidatively modified in norleucine-substituted cells, consistent with loss of the antioxidant defense provided by methionine residues. The results of these studies support the hypothesis that methionine in protein acts as an endogenous antioxidant in cells.
Figures



References
-
- Vogt W. Oxidation of methionine residues in proteins: Tools, targets, and reversal. Free Rad Biol Med. 1995;18:93–105. - PubMed
-
- Stadtman E R, Moskovitz J, Berlett B S, Levine R L. Cyclic oxidation and reduction of protein methionine residues is an important antioxidant mechanism. Mol Cell Biochem. 2002;234–235:3–9. - PubMed
-
- Reddy V Y, Pizzo S V, Weiss S J. Functional inactivation and structural disruption of human alpha 2-macroglobulin by neutrophils and eosinophils. J Biol Chem. 1989;264:13801–13809. - PubMed
-
- Reddy V Y, Desrochers P E, Pizzo S V, Gonias S L, Sahakian J A, Levine R L, Weiss S J. Oxidative dissociation of human α2macroglobulin tetramers into dysfunctional dimers. J Biol Chem. 1994;269:4683–4691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

