Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation
- PMID: 18845811
- PMCID: PMC2682347
- DOI: 10.1161/01.RES.0000338496.95579.56
Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation
Abstract
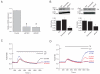
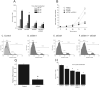
Recent breakthroughs in the store-operated calcium (Ca(2+)) entry (SOCE) pathway have identified Stim1 as the endoplasmic reticulum Ca(2+) sensor and Orai1 as the pore forming subunit of the highly Ca(2+)-selective CRAC channel expressed in hematopoietic cells. Previous studies, however, have suggested that endothelial cell (EC) SOCE is mediated by the nonselective canonical transient receptor potential channel (TRPC) family, TRPC1 or TRPC4. Here, we show that passive store depletion by thapsigargin or receptor activation by either thrombin or the vascular endothelial growth factor activates the same pathway in primary ECs with classical SOCE pharmacological features. ECs possess the archetypical Ca(2+) release-activated Ca(2+) current (I(CRAC)), albeit of a very small amplitude. Using a maneuver that amplifies currents in divalent-free bath solutions, we show that EC CRAC has similar characteristics to that recorded from rat basophilic leukemia cells, namely a similar time course of activation, sensitivity to 2-aminoethoxydiphenyl borate, and low concentrations of lanthanides, and large Na(+) currents displaying the typical depotentiation. RNA silencing of either Stim1 or Orai1 essentially abolished SOCE and I(CRAC) in ECs, which were rescued by ectopic expression of either Stim1 or Orai1, respectively. Surprisingly, knockdown of either TRPC1 or TRPC4 proteins had no effect on SOCE and I(CRAC). Ectopic expression of Stim1 in ECs increased their I(CRAC) to a size comparable to that in rat basophilic leukemia cells. Knockdown of Stim1, Stim2, or Orai1 inhibited EC proliferation and caused cell cycle arrest at S and G2/M phase, although Orai1 knockdown was more efficient than that of Stim proteins. These results are first to our knowledge to establish the requirement of Stim1/Orai1 in the endothelial SOCE pathway.
Figures






Comment in
-
Harmony and discord in endothelial calcium entry.Circ Res. 2009 Jan 30;104(2):e22-3. doi: 10.1161/CIRCRESAHA.108.191338. Circ Res. 2009. PMID: 19179662 Free PMC article. No abstract available.
References
-
- Putney JW., Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. - PubMed
-
- Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW., Jr. Emerging perspectives in store-operated Ca(2+) entry: Roles of Orai, Stim and TRP. Biochim Biophys Acta. 2006 Sep 5; - PubMed
-
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. - PubMed
-
- Parekh AB, Putney JW., Jr. Store-operated calcium channels. Physiol Rev. 2005 Apr;85(2):757–810. - PubMed
-
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006 May 11;441(7090):179–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

