Evaluation of repeat Clostridium difficile enzyme immunoassay testing
- PMID: 18845820
- PMCID: PMC2576601
- DOI: 10.1128/JCM.00931-08
Evaluation of repeat Clostridium difficile enzyme immunoassay testing
Abstract
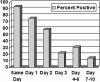
Clostridium difficile is the leading cause of antibiotic-associated diarrhea and pseudomembranous colitis, which have significant morbidity and mortality. Accurate and timely diagnosis is critical. Repeat enzyme immunoassay testing for C. difficile toxin has been recommended because of <100% sensitivity. All C. difficile tests between 1 January 2006 and 31 December 2006 were retrospectively analyzed for results and testing patterns. The Wampole C. difficile Tox A/B II enzyme immunoassay kit was used. There were a total of 8,256 tests from 3,112 patients; 49% of tests were repeated. Of the 3,749 initially negative patient tests, 96 were positive upon repeat testing within 10 days of the first test. Of repeat tests, 0.9% repeated on day 0 (same day as the first test), 1.8% on day 1, 3.8% on day 2, 2.6% on day 3, 5.4% on days 4 to 6, and 10.6% on days 7 to 10 were positive. Thirty-eight patients had a positive test within 48 h of an initial negative test, and based on chart review, 18 patients were treated empirically while 16 were treated following the new result. None had evidence of medical complications. Of initially positive patients, 91% were positive upon repeat testing on day 0, 75% on day 1, and 58% on day 2, to a low of 14% on days 7 to 10. Depending on the clinical setting, these data support not repeating C. difficile tests within 2 days of a negative result and limiting repeat testing to >/=1 week of a positive result.
Figures
References
-
- Aldeen, W. E., M. Bingham, A. Aiderzada, J. Kucera, S. Jense, and K. C. Carroll. 2000. Comparison of the TOX A/B test to a cell culture cytotoxicity assay for the detection of Clostridium difficile in stools. Diagn. Microbiol. Infect. Dis. 36211-213. - PubMed
-
- Aslam, S., and D. M. Musher. 2006. An update on diagnosis, treatment, and prevention of Clostridium difficile-associated disease. Gastroenterol. Clin. N. Am. 35315-335. - PubMed
-
- Bartlett, J. G. 2002. Clinical practice. Antibiotic-associated diarrhea. N. Engl. J. Med. 346334-339. - PubMed
-
- Bartlett, J. G., and D. N. Gerding. 2008. Clinical recognition and diagnosis of Clostridium difficile infection. Clin. Infect. Dis. 46(Suppl. 1)S12-S18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical