Mammalian glycosylation in immunity
- PMID: 18846099
- PMCID: PMC2768770
- DOI: 10.1038/nri2417
Mammalian glycosylation in immunity
Abstract
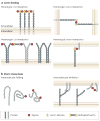
Glycosylation produces a diverse and abundant repertoire of glycans, which are collectively known as the glycome. Glycans are one of the four fundamental macromolecular components of all cells, and are highly regulated in the immune system. Their diversity reflects their multiple biological functions that encompass ligands for proteinaceous receptors known as lectins. Since the discovery that selectins and their glycan ligands are important for the regulation of leukocyte trafficking, it has been shown that additional features of the vertebrate immune system are also controlled by endogenous cellular glycosylation. This Review focuses on the emerging immunological roles of the mammalian glycome.
Figures


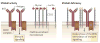
References
-
- Paulson JC, Blixt O, Collins BE. Sweet spots in functional glycomics. Nature Chem Biol. 2006;2:238–248. This publication provides an introduction to the definition and concept of the glycome and describes new approaches to research in this field. - PubMed
-
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. This article reviews the process of glycosylation and the mechanisms by which glycans contribute to molecular interactions that govern health and disease. - PubMed
-
- Raman R, Raguram S, Venkataraman G, Paulson JC, Sasisekharan R. Glycomics: an integrated systems approach to structure–function relationships of glycans. Nature Methods. 2005;2:817–824. - PubMed
-
- Haltiwanger RS, Lowe JB. Role of glycosylation in development. Annu Rev Biochem. 2004;73:491–537. This article reviews the developmental biology of glycosylation and the mechanisms by which glycans control ontogeny. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

