Enhanced oncolytic potency of vesicular stomatitis virus through vector-mediated inhibition of NK and NKT cells
- PMID: 18846115
- PMCID: PMC2924743
- DOI: 10.1038/cgt.2008.74
Enhanced oncolytic potency of vesicular stomatitis virus through vector-mediated inhibition of NK and NKT cells
Abstract
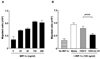
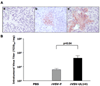
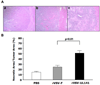
Recombinant oncolytic viruses represent a promising alternative option for the treatment of malignant cancers. We have reported earlier the safety and efficacy of recombinant vesicular stomatitis virus (VSV) vectors in a rat model of hepatocellular carcinoma (HCC). However, the full potential of VSV therapy is limited by a sudden decline in intratumoral virus replication observed early after viral administration, a phenomenon that coincides with an accumulation of inflammatory cells within infected lesions. To overcome the antiviral function of these cells, we present a recombinant virus, rVSV-UL141, which expresses a protein from human cytomegalovirus known to downregulate the natural killer (NK) cell-activating ligand CD155. The modified vector resulted in an inhibition of NK cell recruitment in vitro, as well as decreased intratumoral accumulations of NK and NKT cells in vivo. Administration of rVSV-UL141 through hepatic artery infusion in immune-competent Buffalo rats harboring orthotopic, multi-focal HCC lesions resulted in a one-log elevation of intratumoral virus replication over a control rVSV vector, which translated to enhance tumor necrosis and substantial prolongation of survival. Moreover, these results were achieved in the absence of apparent toxicities. The present study suggests the applicability of this strategy for the development of effective and safe oncolytic agents to treat multi-focal HCC, and potentially a multitude of other cancers, in the future.
Figures





References
-
- Murray CJaL AD. Evidence-based health policy - lessons from the Global Burden of Disease Study. Science. 1996;274(5288):740–743. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. - PubMed
-
- El-Serag HBaM AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340(10):745–750. - PubMed
-
- Dyer Z, Peltekian K, van Zanten SV. The changing epidemiology of hepatocellular carcinoma in Canada. Aliment Pharmacol Ther. 2005;22(1):17–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

