Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury
- PMID: 18846543
- PMCID: PMC2579320
- DOI: 10.1002/hep.22498
Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury
Abstract
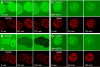
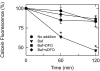
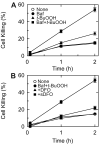
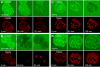
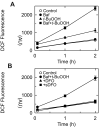
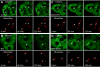
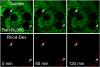
Iron overload exacerbates various liver diseases. In hepatocytes, a portion of non-heme iron is sequestered in lysosomes and endosomes. The precise mechanisms by which lysosomal iron participates in hepatocellular injury remain uncertain. Here, our aim was to determine the role of intracellular movement of chelatable iron in oxidative stress-induced killing to cultured hepatocytes from C3Heb mice and Sprague-Dawley rats. Mitochondrial polarization and chelatable iron were visualized by confocal microscopy of tetramethylrhodamine methylester (TMRM) and quenching of calcein, respectively. Cell viability and hydroperoxide formation (a measure of lipid peroxidation) were measured fluorometrically using propidium iodide and chloromethyl dihydrodichlorofluorescein, respectively. After collapse of lysosomal/endosomal acidic pH gradients with bafilomycin (50 nM), an inhibitor of the vacuolar proton-pumping adenosine triphosphatase, cytosolic calcein fluorescence became quenched. Deferoxamine mesylate and starch-deferoxamine (1 mM) prevented bafilomycin-induced calcein quenching, indicating that bafilomycin induced release of chelatable iron from lysosomes/endosomes. Bafilomycin also quenched calcein fluorescence in mitochondria, which was blocked by 20 microM Ru360, an inhibitor of the mitochondrial calcium uniporter, consistent with mitochondrial iron uptake by the uniporter. Bafilomycin alone was not sufficient to induce mitochondrial depolarization and cell killing, but in the presence of low-dose tert-butylhydroperoxide (25 microM), bafilomycin enhanced hydroperoxide generation, leading to mitochondrial depolarization and subsequent cell death.
Conclusion: Taken together, the results are consistent with the conclusion that bafilomycin induces release of chelatable iron from lysosomes/endosomes, which is taken up by mitochondria. Oxidative stress and chelatable iron thus act as two "hits" synergistically promoting toxic radical formation, mitochondrial dysfunction, and cell death. This pathway of intracellular iron translocation is a potential therapeutic target against oxidative stress-mediated hepatotoxicity.
Figures
References
-
- Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000 Aug 14;149(1):43–50. - PubMed
-
- Starke PE, Farber JL. Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. Evidence for the participation of hydroxyl radicals formed by an iron-catalyzed Haber-Weiss reaction. J Biol Chem. 1985 Aug 25;260(18):10099–10104. - PubMed
-
- Kyle ME, Miccadei S, Nakae D, Farber JL. Superoxide dismutase and catalase protect cultured hepatocytes from the cytotoxicity of acetaminophen. Biochem Biophys Res Commun. 1987 Dec 31;149(3):889–896. - PubMed
-
- Dawson TL, Gores GJ, Nieminen AL, Herman B, Lemasters JJ. Mitochondria as a source of reactive oxygen species during reductive stress in rat hepatocytes. Am J Physiol. 1993 Apr;264(4 Pt 1):C961–C967. - PubMed
-
- Nieminen AL, Byrne AM, Herman B, Lemasters JJ. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD(P)H and reactive oxygen species. Am J Physiol. 1997 Apr;272(4 Pt 1):C1286–C1294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
