Outcome of adults with acute lymphocytic leukemia after second salvage therapy
- PMID: 18846563
- PMCID: PMC4188532
- DOI: 10.1002/cncr.23919
Outcome of adults with acute lymphocytic leukemia after second salvage therapy
Abstract
Background: The outcome of adults with acute lymphocytic leukemia (ALL) who undergo second salvage therapy has been characterized poorly. This is important with regard to investigational approaches aimed at helping this subset of patients. The objectives of the current study were to predict outcomes and determine the prognostic factors associated with second salvage therapy in patients with ALL.
Methods: In this study, 288 patients were analyzed who received second salvage therapy for ALL at the authors' institution.
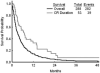
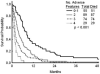
Results: Overall, 53 patients (18%) achieved a complete response (CR). The median remission duration was 7 months and the median survival was 3 months. In multivariate analysis, prognostic factors that were associated independently with achieving CR were duration of first CR and platelet count. Patients with a first CR <36 months and platelet counts <50 x 10(9)/L had an expected CR rate of 7%. In multivariate analysis, prognostic factors that were associated independently with survival were duration of first CR, percentage bone marrow blasts, platelet count, and albumin level. The expected 12-month survival rates for patients with 0 or 1, 2, 3, or 4 adverse factors were 33%, 14%, 8%, and 0%, respectively. A repeat multivariate analysis using landmark assessment at 6 weeks selected achievement of CR as adding significantly to the survival benefit (P = .0001; hazard ratio, 0.51). Only 22 patients (8%) were able to undergo allogeneic stem cell transplantation as second salvage therapy, and their 1-year survival rate was 18%.
Conclusions: The outcome of adults with ALL undergoing second salvage therapy is poor. Novel effective therapies against ALL are needed in this subset of patients.
(c) 2008 American Cancer Society
Figures
References
-
- Pui C-H, Evans WE. Acute lymphoblastic leukemia. N Engl J Med. 1998;339:605–615. - PubMed
-
- Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. - PubMed
-
- Kantarjian H, Thomas D, O’Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. - PubMed
-
- Faderl S, Jeha S, Kantarjian HM. The biology and therapy of adult acute lymphoblastic leukemia. Cancer. 2003;98:1337–1354. - PubMed
-
- Larson RA. Recent clinical trials in acute lymphocytic leukemia by the Cancer and Leukemia Group B. Hematol Oncol Clin North Am. 2000;14:1367–1379. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources