Tricyclic [1,2,4]triazine 1,4-dioxides as hypoxia selective cytotoxins
- PMID: 18847185
- PMCID: PMC2690574
- DOI: 10.1021/jm800967h
Tricyclic [1,2,4]triazine 1,4-dioxides as hypoxia selective cytotoxins
Abstract
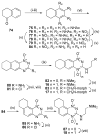
A series of novel tricyclic triazine-di- N-oxides (TTOs) related to tirapazamine have been designed and prepared. A wide range of structural arrangements with cycloalkyl, oxygen-, and nitrogen-containing saturated rings fused to the triazine core, coupled with various side chains linked to either hemisphere, resulted in TTO analogues that displayed hypoxia-selective cytotoxicity in vitro. Optimal rates of hypoxic metabolism and tissue diffusion coefficients were achieved with fused cycloalkyl rings in combination with both the 3-aminoalkyl or 3-alkyl substituents linked to weakly basic soluble amines. The selection was further refined using pharmacokinetic/pharmacodynamic model predictions of the in vivo hypoxic potency (AUC req) and selectivity (HCD) with 12 TTO analogues predicted to be active in vivo, subject to the achievement of adequate plasma pharmacokinetics.
Figures

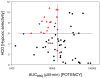
 , active in vivo;
, active in vivo;  , not tested due to structural similarity to actives;
, not tested due to structural similarity to actives;  , not active in vivo. Data from Refs and . Figure 2b. Predicted Hypoxic Selectivity (HCD) and Potency (AUCreq) for TTOs. Legend for Table 2b: ● TTOs from Table 1;
, not active in vivo. Data from Refs and . Figure 2b. Predicted Hypoxic Selectivity (HCD) and Potency (AUCreq) for TTOs. Legend for Table 2b: ● TTOs from Table 1;  , TTOs from Table 2;
, TTOs from Table 2;  , TTOs from Table 3.
, TTOs from Table 3.
 , active in vivo;
, active in vivo;  , not tested due to structural similarity to actives;
, not tested due to structural similarity to actives;  , not active in vivo. Data from Refs and . Figure 2b. Predicted Hypoxic Selectivity (HCD) and Potency (AUCreq) for TTOs. Legend for Table 2b: ● TTOs from Table 1;
, not active in vivo. Data from Refs and . Figure 2b. Predicted Hypoxic Selectivity (HCD) and Potency (AUCreq) for TTOs. Legend for Table 2b: ● TTOs from Table 1;  , TTOs from Table 2;
, TTOs from Table 2;  , TTOs from Table 3.
, TTOs from Table 3.











Similar articles
-
New quinoxaline 1,4-di-N-oxides. Part 1: Hypoxia-selective cytotoxins and anticancer agents derived from quinoxaline 1,4-di-N-oxides.Bioorg Med Chem. 2006 Oct 15;14(20):6917-23. doi: 10.1016/j.bmc.2006.06.038. Epub 2006 Jul 14. Bioorg Med Chem. 2006. PMID: 16843668
-
Synthesis and biological evaluation of 1,2,5-oxadiazole N-oxide derivatives as potential hypoxic cytotoxins and DNA-binders.Arch Pharm (Weinheim). 2000 Nov;333(11):387-93. doi: 10.1002/1521-4184(200011)333:11<387::aid-ardp387>3.0.co;2-n. Arch Pharm (Weinheim). 2000. PMID: 11129981
-
N-oxides as hypoxia selective cytotoxins.Mini Rev Med Chem. 2001 Sep;1(3):219-31. doi: 10.2174/1389557013406891. Mini Rev Med Chem. 2001. PMID: 12369969 Review.
-
Structure-activity relationships of 1,2,4-benzotriazine 1,4-dioxides as hypoxia-selective analogues of tirapazamine.J Med Chem. 2003 Jan 2;46(1):169-82. doi: 10.1021/jm020367+. J Med Chem. 2003. PMID: 12502371
-
Bioreductive prodrugs as cancer therapeutics: targeting tumor hypoxia.Chin J Cancer. 2014 Feb;33(2):80-6. doi: 10.5732/cjc.012.10285. Epub 2013 Jul 12. Chin J Cancer. 2014. PMID: 23845143 Free PMC article. Review.
Cited by
-
Schedule-dependent potentiation of chemotherapy drugs by the hypoxia-activated prodrug SN30000.Cancer Biol Ther. 2019;20(9):1258-1269. doi: 10.1080/15384047.2019.1617570. Epub 2019 May 26. Cancer Biol Ther. 2019. PMID: 31131698 Free PMC article.
-
Isotopic labeling experiments that elucidate the mechanism of DNA strand cleavage by the hypoxia-selective antitumor agent 1,2,4-benzotriazine 1,4-di-N-oxide.Chem Res Toxicol. 2014 Jan 21;27(1):111-8. doi: 10.1021/tx400356y. Epub 2013 Dec 19. Chem Res Toxicol. 2014. PMID: 24328261 Free PMC article.
-
Synthesis and biological evaluation of 3-aryl-quinoxaline-2-carbonitrile 1,4-di-N-oxide derivatives as hypoxic selective anti-tumor agents.Molecules. 2012 Aug 13;17(8):9683-96. doi: 10.3390/molecules17089683. Molecules. 2012. PMID: 22890172 Free PMC article.
-
Redox-directed cancer therapeutics: molecular mechanisms and opportunities.Antioxid Redox Signal. 2009 Dec;11(12):3013-69. doi: 10.1089/ars.2009.2541. Antioxid Redox Signal. 2009. PMID: 19496700 Free PMC article. Review.
-
Development of [131I]I-EOE-TPZ and [131I]I-EOE-TPZMO: Novel Tirapazamine (TPZ)-Based Radioiodinated Pharmaceuticals for Application in Theranostic Management of Hypoxia.Pharmaceuticals (Basel). 2019 Jan 1;12(1):3. doi: 10.3390/ph12010003. Pharmaceuticals (Basel). 2019. PMID: 30609671 Free PMC article.
References
-
- Semenza GL. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenergetics Biomembranes. 2007;39:231–234. - PubMed
-
- Gatenby RA, Gillies RJ. Glycolysis in cancer: a potential target for therapy. Int J Biochem Cell Biol. 2007;39:1358–1366. - PubMed
-
- Harris AL. Hypoxia-a key regulatory factor in tumour growth. Nature Rev Cancer. 2002;2:38–47. - PubMed
-
- Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62–S67. - PubMed
-
- Pennachietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

