Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration
- PMID: 18847330
- PMCID: PMC3276357
- DOI: 10.1359/jbmr.081003
Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration
Erratum in
- J Bone Miner Res. 2009 Apr;24(4):758
Abstract
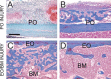
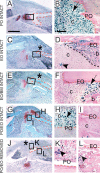
Bone repair requires the mobilization of adult skeletal stem cells/progenitors to allow deposition of cartilage and bone at the injury site. These stem cells/progenitors are believed to come from multiple sources including the bone marrow and the periosteum. The goal of this study was to establish the cellular contributions of bone marrow and periosteum to bone healing in vivo and to assess the effect of the tissue environment on cell differentiation within bone marrow and periosteum. Results show that periosteal injuries heal by endochondral ossification, whereas bone marrow injuries heal by intramembranous ossification, indicating that distinct cellular responses occur within these tissues during repair. [corrected] Next, lineage analyses were used to track the fate of cells derived from periosteum, bone marrow, and endosteum, a subcompartment of the bone marrow. Skeletal progenitor cells were found to be recruited locally and concurrently from periosteum and/or bone marrow/endosteum during bone repair. Periosteum and bone marrow/endosteum both gave rise to osteoblasts, whereas the periosteum was the major source of chondrocytes. Finally, results show that intrinsic and environmental signals modulate cell fate decisions within these tissues. In conclusion, this study sheds light into the origins of skeletal stem cells/progenitors during bone regeneration and indicates that periosteum, endosteum, and bone marrow contain pools of stem cells/progenitors with distinct osteogenic and chondrogenic potentials that vary with the tissue environment.
Figures



References
-
- Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

