Radiation increases invasion of gene-modified mesenchymal stem cells into tumors
- PMID: 18849123
- PMCID: PMC2771691
- DOI: 10.1016/j.ijrobp.2008.06.1953
Radiation increases invasion of gene-modified mesenchymal stem cells into tumors
Abstract
Purpose: Mesenchymal stem cells (MSCs) are multipotent cells in the bone marrow that have been found to migrate to tumors, suggesting a potential use for cancer gene therapy. MSCs migrate to sites of tissue damage, including normal tissues damaged by radiation. In this study, we investigated the effect of tumor radiotherapy on the localization of lentivirus-transduced MSCs to tumors.
Methods and materials: MSCs were labeled with a lipophilic dye to investigate their migration to colon cancer xenografts. Subsequently, the MSCs were transduced with a lentiviral vector to model gene therapy and mark the infused MSCs. LoVo tumor xenografts were treated with increasing radiation doses to assess the effect on MSC localization, which was measured by quantitative polymerase chain reaction. MSC invasion efficiency was determined in an invasion assay.
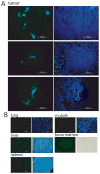
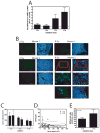
Results: MSCs migrated to tumor xenografts of various origins, with few cells found in normal tissues. A lentiviral vector efficiently transduced MSCs in the presence, but not the absence, of hexadimethrine bromide (Polybrene). When LoVo tumors were treated with increasing radiation doses, more MSCs were found to migrate to them than to untreated tumors. Irradiation increased MSC localization in HT-29 and MDA-MB-231, but not UMSCC1, xenografts. Monocyte chemotactic protein-1 expression in tumors did not correlate with the basal levels of MSC infiltration; however, monocyte chemotactic protein-1 was modestly elevated in irradiated tumors. Media from irradiated LoVo cells stimulated MSC invasion into basement membranes.
Conclusion: These findings suggest that radiation-induced injury can be used to target MSCs to tumors, which might increase the effectiveness of MSC cancer gene therapy. The production of tumor-derived factors in response to radiation stimulates MSC invasion.
Figures




References
-
- Zhang M, Li S, Nyati MK, et al. Regional delivery and selective expression of a high-activity yeast cytosine deaminase in an intrahepatic colon cancer model. Cancer Res. 2003;63:658–663. - PubMed
-
- Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

