Regulation of ACE2 in cardiac myocytes and fibroblasts
- PMID: 18849338
- PMCID: PMC2614534
- DOI: 10.1152/ajpheart.00426.2008
Regulation of ACE2 in cardiac myocytes and fibroblasts
Abstract
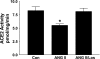
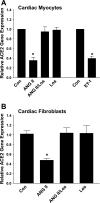
Angiotensin-converting enzyme 2 (ACE2) preferentially forms angiotensin-(1-7) [ANG-(1-7)] from ANG II. We showed that cardiac ACE2 is elevated following treatment of coronary artery-ligated rats with AT1 receptor blockers (ARBs). Cardiac myocytes and fibroblasts were isolated from neonatal rats to determine the molecular mechanisms for the ACE2 upregulation by ARB treatment. ANG II significantly reduced ACE2 activity and downregulated ACE2 mRNA in cardiac myocytes, effects blocked by the ARB losartan, indicating that ANG II regulates ACE2. ANG II also reduced ACE2 mRNA in cardiac fibroblasts; however, no enzyme activity was detected, reflecting the limited expression of ACE2 in these cells. Endothelin-1 (ET-1) also significantly reduced myocyte ACE2 mRNA. The reduction in ACE2 mRNA by ANG II or ET-1 was blocked by inhibitors of mitogen-activated protein kinase kinase 1, suggesting that ANG II or ET-1 activates extracellular signal-regulated kinase (ERK) 1/ERK2 to reduce ACE2. Although ACE2 mRNA was not affected by ANG-(1-7), both the ANG II- and ET-1-mediated reductions in ACE2 mRNA were blocked by the heptapeptide. The ANG-(1-7) modulatory effect was prevented by the ANG-(1-7) receptor antagonist [D-Ala7]-ANG-(1-7), indicating that the ANG-(1-7) response was mediated by a specific AT(1-7) receptor. Myocyte treatment with atrial natriuretic peptide (ANP) also reversed the ACE2 mRNA downregulation by ANG II or ET-1, whereas treatment with ANP alone was ineffective. These results indicate that multiple hypertrophic and anti-hypertropic peptides regulate ACE2 production in myocytes, suggesting that ACE2 expression in the heart is dependent upon the compliment and concentration of regulatory molecules.
Figures




References
-
- Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS, Lu S, Tikellis C, Grant SL, Lew RA, Smith AI, Cooper ME, Johnston CI. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J 114: 1–7, 2005. - PubMed
-
- Chappell MC, Pirro NT, Sykes A, Ferrario CM. Metabolism of angiotensin-(1-7) by angiotensin-converting enzyme. Hypertension 31: 362–367, 1998. - PubMed
-
- Crackower MA, Sarao ROG, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos A, da Costa JZL, Pei YP, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yogil Y, Penninger JM. Angiotensin-converting enzme 2 is an essential regulator of heart function. Nature 417: 822–828, 2002. - PubMed
-
- de Jonge HW, Dekkers DH, Houtsmuller AB, Sharma HS, Lamers JM. Differential signaling and hypertrophic responses in cyclical endothelin-1 stimulated neonatal rat cardiomyocytes. Cell Biochem Biophys 47: 21–32, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

