Na-K-Cl Cotransporter-1 in the mechanism of ammonia-induced astrocyte swelling
- PMID: 18849345
- PMCID: PMC2590687
- DOI: 10.1074/jbc.M804016200
Na-K-Cl Cotransporter-1 in the mechanism of ammonia-induced astrocyte swelling
Abstract
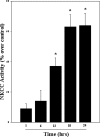
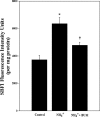
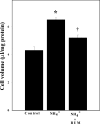
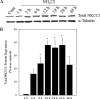
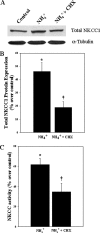
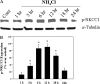
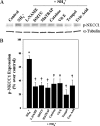
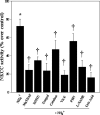
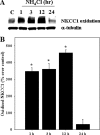
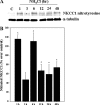
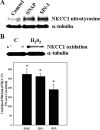
Brain edema and the consequent increase in intracranial pressure and brain herniation are major complications of acute liver failure (fulminant hepatic failure) and a major cause of death in this condition. Ammonia has been strongly implicated as an important factor, and astrocyte swelling appears to be primarily responsible for the edema. Ammonia is known to cause cell swelling in cultured astrocytes, although the means by which this occurs has not been fully elucidated. A disturbance in one or more of these systems may result in loss of ion homeostasis and cell swelling. In particular, activation of the Na-K-Cl cotransporter (NKCC1) has been shown to be involved in cell swelling in several neurological disorders. We therefore examined the effect of ammonia on NKCC activity and its potential role in the swelling of astrocytes. Cultured astrocytes were exposed to ammonia (NH(4)Cl; 5 mm), and NKCC activity was measured. Ammonia increased NKCC activity at 24 h. Inhibition of this activity by bumetanide diminished ammonia-induced astrocyte swelling. Ammonia also increased total as well as phosphorylated NKCC1. Treatment with cyclohexamide, a potent inhibitor of protein synthesis, diminished NKCC1 protein expression and NKCC activity. Since ammonia is known to induce oxidative/nitrosative stress, and antioxidants and nitric-oxide synthase inhibition diminish astrocyte swelling, we also examined whether ammonia caused oxidation and/or nitration of NKCC1. Cultures exposed to ammonia increased the state of oxidation and nitration of NKCC1, whereas the antioxidants N-nitro-l-arginine methyl ester and uric acid all significantly diminished NKCC activity. These agents also reduced phosphorylated NKCC1 expression. These results suggest that activation of NKCC1 is an important factor in the mediation of astrocyte swelling by ammonia and that such activation appears to be mediated by NKCC1 abundance as well as by its oxidation/nitration and phosphorylation.
Figures

References
-
- Vaquero, J., Chung, C., and Blei, A. T. (2003) Ann. Hepatol. 2 12–22 - PubMed
-
- Albrecht, J., and Jones, E. A. (1999) J. Neurol. Sci. 170 138–146 - PubMed
-
- Hazell, A. S., and Butterworth, R. F. (1999) Proc. Soc. Exp. Biol. Med. 222 99–112 - PubMed
-
- Martinez, A. J. (1968) Acta Neuropathol. (Berl.) 11 82–86 - PubMed
-
- Norenberg, M. D. (1977) Lab. Invest. 36 618–627 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

