A novel role for activating transcription factor-2 in 15(S)-hydroxyeicosatetraenoic acid-induced angiogenesis
- PMID: 18849464
- PMCID: PMC2638108
- DOI: 10.1194/jlr.M800388-JLR200
A novel role for activating transcription factor-2 in 15(S)-hydroxyeicosatetraenoic acid-induced angiogenesis
Abstract
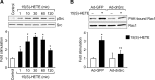
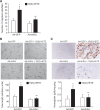
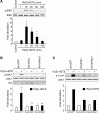
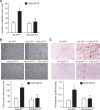
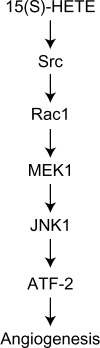
To investigate the mechanisms underlying 15(S)-HETE-induced angiogenesis, we have studied the role of the small GTPase, Rac1. We find that 15(S)-HETE activated Rac1 in human retinal microvascular endothelial cells (HRMVEC) in a time-dependent manner. Blockade of Rac1 by adenovirus-mediated expression of its dominant negative mutant suppressed HRMVEC migration as well as tube formation and Matrigel plug angiogenesis. 15(S)-HETE stimulated Src in HRMVEC in a time-dependent manner and blockade of its activation inhibited 15(S)-HETE-induced Rac1 stimulation in HRMVEC and the migration and tube formation of these cells as well as Matrigel plug angiogenesis. 15(S)-HETE stimulated JNK1 in Src-Rac1-dependent manner in HRMVEC and adenovirus-mediated expression of its dominant negative mutant suppressed the migration and tube formation of these cells and Matrigel plug angiogenesis. 15(S)-HETE activated ATF-2 in HRMVEC in Src-Rac1-JNK1-dependent manner and interference with its activation via adenovirus-mediated expression of its dominant negative mutant abrogated migration and tube formation of HRMVEC and Matrigel plug angiogenesis. In addition, 15(S)-HETE-induced MEK1 stimulation was found to be dependent on Src-Rac1 activation. Blockade of MEK1 activation inhibited 15(S)-HETE-induced JNK1 activity and ATF-2 phosphorylation. Together, these findings show that 15(S)-HETE activates ATF-2 via the Src-Rac1-MEK1-JNK1 signaling axis in HRMVEC leading to their angiogenic differentiation.
Figures





References
-
- Sigal E., C. S. Craik, E. Highland, D. Grunberger, L. L. Costello, R. A. Dixon, and J. A. Nadel. 1988. Molecular cloning and primary structure of human 15-lipoxygenase. Biochem. Biophys. Res. Commun. 157 457–464. - PubMed
-
- Bryant R. W., J. M. Bailey, T. Schewe, and S. M. Rapoport. 1982. Positional specificity of a reticulocyte lipoxygenase. Conversion of arachidonic acid to 15(S)-hydroperoxyeicosatetraenoic acid. J. Biol. Chem. 257 6050–6055. - PubMed
-
- Kilty L., A. Logan, and P. J. Vickers. 1999. Differential characteristics of human 15-lipoxygenase isozymes and a novel splice variant of 15S-lipoxygenase. Eur. J. Biochem. 266 83–93. - PubMed
-
- Chang M. S., C. Schneider, R. L. Roberts, S. B. Shappell, F. R. Haselton, W. E. Boeglin, and A. R. Brash. 2005. Detection and subcellular localization of two 15S-lipoxygenases in human cornea. Invest. Ophthalmol. Vis. Sci. 46 849–856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

