Pbx1 functions in distinct regulatory networks to pattern the great arteries and cardiac outflow tract
- PMID: 18849531
- PMCID: PMC2680673
- DOI: 10.1242/dev.022350
Pbx1 functions in distinct regulatory networks to pattern the great arteries and cardiac outflow tract
Abstract
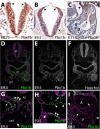
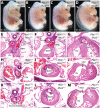
The patterning of the cardiovascular system into systemic and pulmonic circulations is a complex morphogenetic process, the failure of which results in clinically important congenital defects. This process involves extensive vascular remodeling and coordinated division of the cardiac outflow tract (OFT). We demonstrate that the homeodomain transcription factor Pbx1 orchestrates separate transcriptional pathways to control great-artery patterning and cardiac OFT septation in mice. Pbx1-null embryos display anomalous great arteries owing to a failure to establish the initial complement of branchial arch arteries in the caudal pharyngeal region. Pbx1 deficiency also results in the failure of cardiac OFT septation. Pbx1-null embryos lose a transient burst of Pax3 expression in premigratory cardiac neural crest cells (NCCs) that ultimately specifies cardiac NCC function for OFT development, but does not regulate NCC migration to the heart. We show that Pbx1 directly activates Pax3, leading to repression of its target gene Msx2 in NCCs. Compound Msx2/Pbx1-null embryos display significant rescue of cardiac septation, demonstrating that disruption of this Pbx1-Pax3-Msx2 regulatory pathway partially underlies the OFT defects in Pbx1-null mice. Conversely, the great-artery anomalies of compound Msx2/Pbx1-null embryos remain within the same spectrum as those of Pbx1-null embryos. Thus, Pbx1 makes a crucial contribution to distinct regulatory pathways in cardiovascular development.
Figures






References
-
- Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development. 2002;129:4613–4625. - PubMed
-
- Arnold JS, Werling U, Braunstein EM, Liao J, Nowotschin S, Edelmann W, Hebert JM, Morrow BE. Inactivation of Tbx1 in the pharyngeal endoderm results in 22q11DS malformations. Development. 2006;133:977–987. - PubMed
-
- Brendolan A, Ferretti E, Salsi V, Moses K, Quaggin S, Blasi F, Cleary ML, Selleri L. A Pbx1-dependent genetic and transcriptional network regulates spleen ontogeny. Development. 2005;132:3113–3126. - PubMed
-
- Brown CB, Feiner L, Lu MM, Li J, Ma X, Webber AL, Jia L, Raper JA, Epstein JA. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development. 2001;128:3071–3080. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

