Active cystathionine beta-synthase can be expressed in heme-free systems in the presence of metal-substituted porphyrins or a chemical chaperone
- PMID: 18849566
- PMCID: PMC2596375
- DOI: 10.1074/jbc.M805928200
Active cystathionine beta-synthase can be expressed in heme-free systems in the presence of metal-substituted porphyrins or a chemical chaperone
Abstract
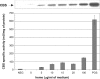
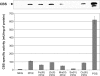
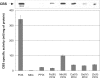
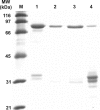
Cystathionine beta-synthase (CBS), a key enzyme in the metabolism of homocysteine, has previously been shown to require a heme co-factor for maximal activity. However, the biochemical function of the CBS heme is not well defined. Here, we show that expression of human CBS in heme-deficient strains of Saccharomyces cerevisiae and Escherichia coli results in production of an enzyme that is misfolded and degraded. Addition of exogenous heme, porphyrins with non-iron metal, or porphyrin lacking metal entirely produced stable and active CBS enzyme. Purification of recombinant CBS enzyme expressed in the presence of various metalloporphyrins confirmed that Mn(III) and Co(III) had 30-60% of the specific activity of Fe(III)-CBS, and still responded to allosteric activation by S-adenosyl-L-methionine. Treatment of S. cerevisiae with the chemical chaperone trimethylamine-N-oxide resulted in near complete restoration of function to human CBS produced in a heme-deficient strain. Taken together, these results suggest that porphyrin moiety of the heme plays a critical role in proper CBS folding and assembly, but that the metal ion is not essential for this function or for allosteric regulation by S-adenosyl-L-methionine.
Figures

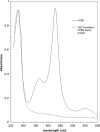
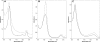
References
-
- Mudd, S. H., Levy, H. L., and Kraus, J. P. (2001) in The Metabolic and Molecular Bases of Inherited Disease (Scriver, C. R., Beaudet, A. L., Sly, W. S., Valle, D., Childs, B., Kinzler, K., and Vogelstein, B., eds) pp. 2007–2056, 8 Ed., McGraw-Hill, New York
-
- Stampfer, M. J., Malinow, M. R., Willett, W. C., Newcomer, L. M., Upson, B., Ullmann, D., Tishler, P. V., and Hennekens, C. H. (1992) J. Am. Med. Assoc. 268 877–881 - PubMed
-
- Mills, J. L., Scott, J. M., Kirke, P. N., McPartlin, J. M., Conley, M. R., Weir, D. G., Molloy, A. M., and Lee, Y. J. (1996) J. Nutr. 126 756S–760S - PubMed
-
- Seshadri, S., Beiser, A., Selhub, J., Jacques, P. F., Rosenberg, I. H., D'Agostino, R. B., Wilson, P. W., and Wolf, P. A. (2002) N. Engl. J. Med. 346 476–483 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

