The ion pathway through the opened Na(+),K(+)-ATPase pump
- PMID: 18849964
- PMCID: PMC2585603
- DOI: 10.1038/nature07350
The ion pathway through the opened Na(+),K(+)-ATPase pump
Abstract
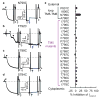
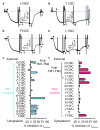
P-type ATPases pump ions across membranes, generating steep electrochemical gradients that are essential for the function of all cells. Access to the ion-binding sites within the pumps alternates between the two sides of the membrane to avoid the dissipation of the gradients that would occur during simultaneous access. In Na(+),K(+)-ATPase pumps treated with the marine agent palytoxin, this strict alternation is disrupted and binding sites are sometimes simultaneously accessible from both sides of the membrane, transforming the pumps into ion channels (see, for example, refs 2, 3). Current recordings in these channels can monitor accessibility of introduced cysteine residues to water-soluble sulphydryl-specific reagents. We found previously that Na(+),K(+) pump-channels open to the extracellular surface through a deep and wide vestibule that emanates from a narrower pathway between transmembrane helices 4 and 6 (TM4 and TM6). Here we report that cysteine scans from TM1 to TM6 reveal a single unbroken cation pathway that traverses palytoxin-bound Na(+),K(+) pump-channels from one side of the membrane to the other. This pathway comprises residues from TM1, TM2, TM4 and TM6, passes through ion-binding site II, and is probably conserved in structurally and evolutionarily related P-type pumps, such as sarcoplasmic- and endoplasmic-reticulum Ca(2+)-ATPases and H(+),K(+)-ATPases.
Figures


References
-
- Läuger P. Electrogenic Ion Pumps. Sinauer; Sunderland, MA: 1991.
-
- Scheiner-Bobis G, Meyer zu Heringdorf D, Christ M, Habermann E. Palytoxin induces K+ efflux from yeast cells expressing the mammalian sodium pump. Mol Pharmacol. 1994;45:1132–1136. - PubMed
-
- Karlin A, Akabas MH. Substituted-cysteine accessibility method. Methods Enzymol. 1998;293:123–145. - PubMed
-
- Reyes N, Gadsby DC. Ion Permeation through the Na+,K+-ATPase. Nature. 2006;443:470–474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

