Adverse effects of vitamin E by induction of drug metabolism
- PMID: 18850180
- PMCID: PMC2474942
- DOI: 10.1007/s12263-007-0055-0
Adverse effects of vitamin E by induction of drug metabolism
Abstract
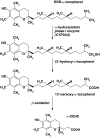
Observational studies with healthy persons demonstrated an inverse association of vitamin E with the risk of coronary heart disease or cancer, the outcome of large-scale clinical trials conducted to prove a benefit of vitamin E in the recurrence and/or progression of such disease, however, was disappointing. Vitamin E did not provide benefits to patients with cardiovascular diseases, cancer, diabetes or hypertension. Even harmful events and worsening of pre-existing diseases were reported, which are hard to explain. Since vitamin E is metabolized along the same routes as xenobiotics and induces drug-metabolizing enzymes in rodents, it is hypothesized that a supplementation with high dosages of vitamin E may also lead to an induction of the drug-metabolizing system in patients that depend on drug therapy. Compromising essential therapy might therefore outweigh any benefit of vitamin E in patients. It is recommended to work out at which threshold the drug-metabolizing system can be induced in humans before new trials with high dosages of vitamin E are started.
Figures
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1081/JDI-200048225', 'is_inner': False, 'url': 'https://doi.org/10.1081/jdi-200048225'}, {'type': 'PubMed', 'value': '15807185', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15807185/'}]}
- Antoniadi G, Eleftheriadis T, Liakopoulos V, Kakasi E, Vayonas G, Kortsaris A, Vargemezis V (2005) Effect of 1 year oral alpha-Tocopherol administration on anticardiolipin antibodies in heamodialysis patients. Ren Fail 27:193–198 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1056/NEJM199405193302007', 'is_inner': False, 'url': 'https://doi.org/10.1056/nejm199405193302007'}, {'type': 'PubMed', 'value': '8127329', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8127329/'}]}
- ATBC prevention study group (1994) The effect of vitamin E and beta-Carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 330:1029–1035 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '16027468', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16027468/'}]}
- Baggott JE (2005) Comment on: high-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med 143:155–156 author reply 156–158 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0891-5849(01)00574-3', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0891-5849(01)00574-3'}, {'type': 'PubMed', 'value': '11440834', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11440834/'}]}
- Birringer M, Drogan D, Brigelius-Flohé R (2001) Tocopherols are metabolized in HepG2 cells by side chain (omega oxidation and consecutive beta-oxidation. Free Radic Biol Med 31:226–232 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '12368403', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12368403/'}]}
- Birringer M, Pfluger P, Kluth D, Landes N, Brigelius-Flohé R (2002) Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J Nutr 132:3113–3118 - PubMed
LinkOut - more resources
Full Text Sources
