The interactions of flavonoids within neuronal signalling pathways
- PMID: 18850181
- PMCID: PMC2474943
- DOI: 10.1007/s12263-007-0056-z
The interactions of flavonoids within neuronal signalling pathways
Abstract
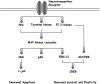
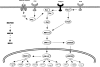
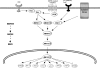
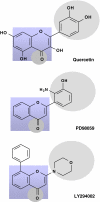
Emerging evidence suggests that dietary phytochemicals, in particular flavonoids, may exert beneficial effects in the central nervous system by protecting neurons against stress-induced injury, by suppressing neuroinflammation and by promoting neurocognitive performance, through changes in synaptic plasticity. It is likely that flavonoids exert such effects in neurons, through selective actions on different components within a number of protein kinase and lipid kinase signalling cascades, such as phosphatidylinositol-3 kinase (PI3K)/Akt, protein kinase C and mitogen-activated protein kinase. This review details the potential inhibitory or stimulatory actions of flavonoids within these pathways, and describes how such interactions are likely to affect cellular function through changes in the activation state of target molecules and/or by modulating gene expression. Although, precise sites of action are presently unknown, their abilities to: (1) bind to ATP binding sites on enzymes and receptors; (2) modulate the activity of kinases directly; (3) affect the function of important phosphatases; (4) preserve neuronal Ca(2+) homeostasis; and (5) modulate signalling cascades lying downstream of kinases, are explored. Future research directions are outlined in relation to their precise site(s) of action within the signalling pathways and the sequence of events that allow them to regulate neuronal function in the central nervous system.
Figures

References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '7562582', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/7562582/'}]}
- Aasmundstad TA, Morland J, Paulsen RE (1995) Distribution of morphine 6-glucuronide and morphine across the blood–brain barrier in awake, freely moving rats investigated by in vivo microdialysis sampling. J Pharmacol Exp Ther 275:435–441 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC1570746', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC1570746/'}, {'type': 'PubMed', 'value': '12162730', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12162730/'}]}
- Abbott NJ (2002) Astrocyte–endothelial interactions and blood–brain barrier permeability. J Anat 200:629–638 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '12488137', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12488137/'}]}
- Abd El Mohsen MM, Kuhnle G, Rechner AR, Schroeter H, Rose S, Jenner P, Rice-Evans CA (2002) Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med 33:1693–1702 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '16457806', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16457806/'}]}
- Adachi N, Tomonaga S, Tachibana T, Denbow DM, Furuse M (2006) (−)-Epigallocatechin gallate attenuates acute stress responses through GABAergic system in the brain. Eur J Pharmacol 531:171–175 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC1171222', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC1171222/'}, {'type': 'PubMed', 'value': '10064598', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10064598/'}]}
- Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, Davis RJ, Ronai Z (1999a) Regulation of JNK signaling by GSTp. EMBO J 18:1321–1334 - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
