Total synthesis of the originally assigned structure of vannusal B
- PMID: 18850598
- PMCID: PMC2790063
- DOI: 10.1002/anie.200804228
Total synthesis of the originally assigned structure of vannusal B
Figures
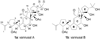
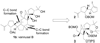
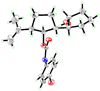
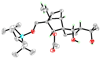

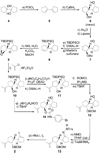
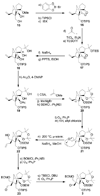
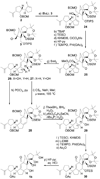
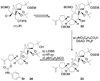
References
-
- Guella G, Callone E, Di Giuseppe G, Frassanito R, Frontini FP, Mancini I, Dini F. Eur. J. Org. Chem. 2007:5226–5234.
-
- Griedinger DS, Ginsburg D. J. Org. Chem. 1957;22:1406–1410.
-
-
For reviews on enzymatic desymmetrization in organic synthesis, see Garcia-Urdiales E, Alfoso I, Gotor V. Chem. Rev. 2005;105:313–354. Hartung IV, Hoffmann HMR. Angew. Chem. 2004;116:1968–1984. Angew. Chem. Int. Ed. 2004;43:1934–1949.
-
-
- Martin SF, Dodge JF. Tetrahedron Lett. 1991;32:3017–3020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

