Development and validation of a French patient-based health-related quality of life instrument in kidney transplant: the ReTransQoL
- PMID: 18851730
- PMCID: PMC2577632
- DOI: 10.1186/1477-7525-6-78
Development and validation of a French patient-based health-related quality of life instrument in kidney transplant: the ReTransQoL
Abstract
Background: In the absence of a French health-related quality of life (QOL) instrument for renal transplant recipients (RTR), we developed a self-administered questionnaire: the ReTransQol (RTQ).
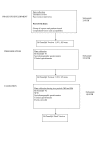
Methods: This questionnaire was developed using classical methodology in the following three phases over a two-year period: Item Generation phase, identifying all possible items having adverse impact on the QOL of RTR, Item Reduction phase, selecting the most pertinent items related to QOL, and Validation phase, analyzing the psychometric properties. All RTR involved in these phases were over 18 and were randomly selected from a transplant registry.
Results: Item generation was conducted through 24 interviews of RTR. The first version of RTQ (85 items) was sent to 225 randomized RTR, and 40 items were eliminated at the end of the item reduction phase. The second version of RTQ (45 items) was validated from 130 RTR, resulting in the RTQ final version. The factor analysis identified a structure of five factors: Physical Health (PH), Mental Health (MH), Medical Care (MC), Fear of losing the Graft (FG) and Treatment (TR). The psychometric properties of RTQ were satisfactory. Comparison between known groups from the literature confirmed the construct validity: patients without employment or living alone have lower QOL scores, and women have lower QOL scores than men. RTQ was more responsive than SF36 to detect changes in the QOL of RTR who were hospitalized secondary to their renal disease in the 4 weeks preceding their inclusion.
Conclusion: According to French public health priorities, RTQ appears to be a reliable and valid questionnaire.
Figures
References
-
- Spiker BN. Quality of life and phamarcoeconomics in clinical trials. 2. Philadelphia, NY: Lippincott-Raven; 1996.
-
- Ganz PA. Impact of quality of life outcomes on clinical practice. Oncology. 1995;11:61–65. - PubMed
-
- Stewart AL, Hays RD, Ware JE. Methods of validating MOS health Measures. In: Stewart AL, Ware JE, Durham, editor. Measuring functioning and well-being: the medical outcomes study approach. NC: Duke University Press; 1992. pp. 309–324.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical