Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity
- PMID: 18851874
- PMCID: PMC2710968
- DOI: 10.1016/j.jaci.2008.08.018
Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity
Abstract
Background: Human hypomorphic nuclear factor-kappaB essential modulator (NEMO) mutations cause diverse clinical and immunologic phenotypes, but understanding of their scope and mechanistic links to immune function and genotype is incomplete.
Objective: We created and analyzed a database of hypomorphic NEMO mutations to determine the spectrum of phenotypes and their associated genotypes and sought to establish a standardized NEMO reconstitution system to obtain mechanistic insights.
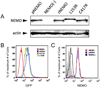
Methods: Phenotypes of 72 individuals with NEMO mutations were compiled. NEMO L153R and C417R were investigated further in a reconstitution system. TNF-alpha or Toll-like receptor (TLR)-5 signals were evaluated for nuclear factor-kappaB activation, programmed cell death, and A20 gene expression.
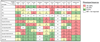
Results: Thirty-two different mutations were identified; 53% affect the zinc finger domain. Seventy-seven percent were associated with ectodermal dysplasia, 86% with serious pyogenic infection, 39% with mycobacterial infection, 19% with serious viral infection, and 23% with inflammatory diseases. Thirty-six percent of individuals died at a mean age of 6.4 years. CD40, IL-1, TNF-alpha, TLR, and T-cell receptor signals were impaired in 15 of 16 (94%), 6 of 7 (86%), 9 of 11 (82%), 9 of 14 (64%), and 7 of 18 (39%), respectively. Hypomorphism-reconstituted NEMO-deficient cells demonstrated partial restoration of NEMO functions. Although both L153R and C417R impaired TLR and TNF-alpha-induced NF-kappaB activation, L153R also increased TNF-alpha-induced programmed cell death with decreased A20 expression.
Conclusion: Distinct NEMO hypomorphs define specific disease and genetic characteristics. A reconstitution system can identify attributes of hypomorphisms independent of an individual's genetic background. Apoptosis susceptibility in L153R reconstituted cells defines a specific phenotype of this mutation that likely contributes to the excessive inflammation with which it is clinically associated.
Figures
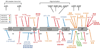


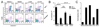
References
-
- Aradhya S, Bardaro T, Galgoczy P, Yamagata T, Esposito T, Patlan H, et al. Multiple pathogenic and benign genomic rearrangements occur at a 35 kb duplication involving the NEMO and LAGE2 genes. Hum Mol Genet. 2001;10:2557–2567. - PubMed
-
- Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. - PubMed
-
- Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27:277–285. - PubMed
-
- Jain A, Ma CA, Liu S, Brown M, Cohen J, Strober W. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol. 2001;2:223–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

