Abnormal metabolism of glycogen phosphate as a cause for Lafora disease
- PMID: 18852261
- PMCID: PMC2590708
- DOI: 10.1074/jbc.M807428200
Abnormal metabolism of glycogen phosphate as a cause for Lafora disease
Abstract
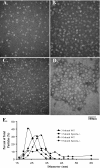
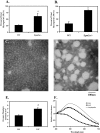
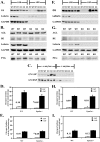
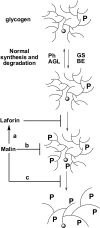
Lafora disease is a progressive myoclonus epilepsy with onset in the teenage years followed by neurodegeneration and death within 10 years. A characteristic is the widespread formation of poorly branched, insoluble glycogen-like polymers (polyglucosan) known as Lafora bodies, which accumulate in neurons, muscle, liver, and other tissues. Approximately half of the cases of Lafora disease result from mutations in the EPM2A gene, which encodes laforin, a member of the dual specificity protein phosphatase family that is able to release the small amount of covalent phosphate normally present in glycogen. In studies of Epm2a(-/-) mice that lack laforin, we observed a progressive change in the properties and structure of glycogen that paralleled the formation of Lafora bodies. At three months, glycogen metabolism remained essentially normal, even though the phosphorylation of glycogen has increased 4-fold and causes altered physical properties of the polysaccharide. By 9 months, the glycogen has overaccumulated by 3-fold, has become somewhat more phosphorylated, but, more notably, is now poorly branched, is insoluble in water, and has acquired an abnormal morphology visible by electron microscopy. These glycogen molecules have a tendency to aggregate and can be recovered in the pellet after low speed centrifugation of tissue extracts. The aggregation requires the phosphorylation of glycogen. The aggregrated glycogen sequesters glycogen synthase but not other glycogen metabolizing enzymes. We propose that laforin functions to suppress excessive glycogen phosphorylation and is an essential component of the metabolism of normally structured glycogen.
Figures



References
-
- Roach, P. J. (2002) Curr. Mol. Med. 2 101–120 - PubMed
-
- Ball, S., Guan, H. P., James, M., Myers, A., Keeling, P., Mouille, G., Buleon, A., Colonna, P., and Preiss, J. (1996) Cell 86 349–352 - PubMed
-
- Gunja-Smith, Z., Marshall, J. J., Mercier, C., Smith, E. E., and Whelan, W. J. (1970) FEBS Lett. 12 101–104 - PubMed
-
- Shearer, J., and Graham, T. E. (2004) Exerc. Sport Sci. Rev. 32 120–126 - PubMed
-
- Lomako, J., Lomako, W. M., Whelan, W. J., and Marchase, R. B. (1993) FEBS Lett. 329 263–267 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

